Note: if you’re a stickler for video quality, watch this on Youtube instead! The max video size for Substack is 20 gigabytes, and this video, sadly, came out to 20.5G. I had to mess with alternative video codecs (H.264 → H.265) to get it smaller. This shouldn’t have strongly noticeable differences, but maybe you have a keener eye than I do. The original quality video is on Youtube!
Introduction
There's a lot of discussion these days on how China's biotech market is on track to bypass the US's. I wondered: shouldn't we have observed the exact same phenomenon with India? It has seemingly all the same ingredients: low cost of labor, smart people, and a massive internal market.
Yet, the Indian biotech research scene is nearly nonexistent. Why is that?
To figure it out, I had a two-hour discussion with Soham Sankaran, the founder and CEO of PopVax, an mRNA vaccine development startup. Amongst those in the know, Soham is well understood as one of the most talented biotech founders in India, and his company has had a genuinely incredible underdog success story. This story is still being written, but there's good reason to be bullish, given that PopVax has an (in mouse) influenza vaccine that is 250x better than its competitors, multiple large research collaborations, and their first upcoming US based phase 1 clinical trial being fully sponsored and conducted by the NIH.
We discuss so many things. Including policy prescriptions for Indian R&D, why PopVax's vaccines are so good, how machine-learning is changing vaccine development, and much more. Transcript below, and links in thread (including a jargon explanation).
Timestamps and transcripts are below. Just as in my last episode, I’ve included a ‘jargon explanation’ as a quick primer for some of the subjects discussed in the episode.
Some final bits: the studio rental costs were kindly covered by Dylan Reid at Zetta Partners! Huge shout-out to him for making this episode possible. Also shout-out to Samarth Jajoo, Reha Mathur, and David Yang for some very helpful discussion about the Indian biotech scene. And, if you think PopVax is interesting, here is their Substack which has some articles on their results, their job section (they are actively hiring), and can be reached at contact@popvax.com.
Jargon explanation
Antigen versus immunogen: This is explained again in the podcast, but, concretely: the antigen target you want antibodies to recognize, like a viral protein or bacterial toxin. An immunogen is what you actually put in the vaccine to generate those antibodies. Just because something can be bound by antibodies (antigen) doesn't mean it will generate good antibodies when used in a vaccine (immunogen). This can be for a lot of reasons, which is discussed in the episode.
Epitope: The specific part of an antigen (or immunogen) that an antibody recognizes and binds to.
Antibody elicitation is the process of getting your immune system to produce specific antibodies. When you give someone a vaccine, you want it to "elicit" (cause the production of) antibodies that can recognize and fight off the target pathogen. The challenging part, as Soham explains in the interview, is that just because something can bind to an antibody doesn't mean it will cause your body to make that antibody when used as a vaccine.
This is why vaccine design is so tricky. You need to carefully design your vaccine to elicit the right kinds of antibodies. Remember, your adaptive immune system will undergo affinity maturation to adapt to whatever immunogen it sees, it won’t simply produce something that binds well! Your immunogen is simply a guiding force in this stochastic, antibody evolution process, and it must lead that process down the right pathway for good antibodies to be bad.
Effector function refers to how antibodies actually fight pathogens beyond just binding to them. In antibody structure lingo, it specifically refers to the the ‘Fc’ part of this diagram. It is, for the most part, constant and doesn’t interact with the pathogen itself, but rather the rest of the immune system.
How does this effect immune design? You need the antibody elicitation caused by your chosen immunogen to not only bind well to the immunogen, but also, one, be a type of antibody that has a ‘desirable’ Fc region (e.g. IgG1, IgG2, IgG3, IgG4 have different Fc regions), and two, have the antibodies cluster around the immunogen densely enough to activate the immune system.
A good case of not taking effector function into account is mentioned by Soham: HSV-2, where vaccines focused only on neutralization failed because effector functions turned out to be crucial for protection.
Timestamps
01:31 Introduction
02:38 Why is there such little biotech research in India?
17:43 Advantages of building a company in India
31:30 Policy prescriptions for India
35:39 Questions on vaccine design
50:55 What does PopVax do?
01:01:58 The role of machine learning in vaccine design
01:12:07 The (conservative) culture of vaccinology
01:26:57 Hiring in India
01:46:52 How fundraising for an Indian vaccine design startup is coming along
01:57:36 How is PopVax so good at designing vaccines?
02:02:07 Pet theories on immune mechanisms
02:09:07 mRNA beyond infectious diseases
02:12:38 What would you do with $100 million dollars?
Transcript
[00:00:00] Preview
[00:00:00] Soham: I think fundamentally, there's a problem of cowardice in India, right? In our elites. Our elites talk a big game. Increasingly, they talk a big geopolitical game as well. But when it comes to actually doing difficult and risky things, we tend to shrink away from it
I think it's an important distinction because there's lots of immunologists.
Not that many vaccinologists, right? People actually practically working on the problem of better vaccines, fewer than you would think. Lots of, like, people working on, like, basic science immunology, systems immunology, increasingly. People actually working on practical, like, nuts and bolts vaccine design, not so many.
We can find parts of the design space that maybe haven't been tested before by other people that in combination you get these really good results.
[00:00:42] Abhi: Because other people don't have like, don't have like the knobs to tune on those because they're outsourcing it all?
[00:00:47] Soham: Yes, they don't have the knobs to tune and they don't have the wherewithal to test all this different stuff.
[00:00:50] Abhi: Gotcha.
[00:00:51] Soham: Right? And I have all the knobs, right? All the knobs are in my control. And so, and we find lots of wacky stuff, right? We find that there are certain lipid nanoparticle formulations that work better for certain vaccine designs. Mm hmm. Do I know why? No. Is my team trying to figure out why? Yeah, totally.
And that's what we're doing at PopVax to some extent. It's 10 times cheaper for us to do a lot of the wet lab work. It's 10 times cheaper for us to do animal work.
And so that means for the same dollar, I can do either 10 programs or I can potentially go 10 times as fast on the same program, right? And eventually, we will just leapfrog what a company like Moderna is able to do because, you know, they can't keep losing the billions of dollars that they're losing forever.
[00:01:31] Introduction
---
[00:01:31] Abhi: I'm incredibly excited to talk with Soham Sankaran, the founder and CEO of Popvax, an mRNA vaccine development startup that he has ran for the last three and a half years. Soham has an interesting background because it's not at all in biology. He has a bachelor's in computer science from Yale, got partway through a PhD in robotics from Cornell, dropped out to start a software for robotics company, and then decided to pivot to biotech during the height of the COVID pandemic.
Most interesting of all is that unlike almost every other R&D biotech company today, PopVax is not based in the U.S.. Or Europe or East Asia, it is based in India, which as any biotech venture capitalist would tell you is an extremely unlikely place for novel biotech research to be conducted. Yet the company is incredibly successful, having won multiple large research contracts and having their first upcoming U.S. based clinical trial fully conducted and sponsored by the NIH. Congratulations.
[00:02:23] Soham: Thank you.
[00:02:24] Abhi: Today we'll be discussing what has stymied biology research in India, how machine learning is changing vaccine design and questions over PopVax's recent success in vaccine development. Thank you for coming on to the show Soham.
[00:02:36] Soham: Very excited. Thank you for having me.
[00:02:38] Why is there such little biotech research in India?
---
[00:02:38] Abhi: So, like, first question is to start things off. you've mentioned in the past that one of the key things preventing further biotech research in India is an inability to be comfortable with technical risk.
Do you think this is cultural? Something that is entirely built into the psyche of India? Is it downstream of a thousand other other things? And then, is it at all fixable?
[00:02:54] Soham: You know about the, the chaebols in, in South Korea?
Yeah. Yeah. Right. And like, you know, the zaibatsu in, in Japan and so on and so forth. Right. They are these sort of big conglomerates. They do a bunch of different things, right? They're like Samsung's and so on and so forth. Yes, as you said. But, what's, I think, particularly interesting about them is like, they're somewhat similar to businesses that we have in India, right?
So the Tata group that you may be familiar with, right? Reliance, which is also a big conglomerate that started in, textiles, then went into petroleum and now does a bunch of all other things, including, they're the biggest telecom operator in India with Jio and so on and so forth. And there are some emerging groups like the Adani group in India, that are also trying to do a whole bunch of different businesses, right?
So there's some parallels here. Right. And I think the government in India has pushed a strategy of these sort of national champion conglomerates, which are, you know, have, you know, government sanction in some sense to, to do risky things. Right. And just sort of build infrastructure and so on and so forth and, you know, build the country.
Right. And I think that that parallels explicitly the strategy that, that South Korea took, that Japan took, and, you know, to some extent Taiwan as well. Right. The distinction, and this is a very critical distinction, is that those companies, and increasingly in China as well, have invested a lot of money in R&D, right?
So they started by doing more copycat type things, you might say, right? Volume manufacturing or sort of, you know, infrastructure projects that are similar to what happened in the West. But then, you know, companies like Samsung have like a big chemical businesses that do specialty chemicals. They have semiconductor businesses where they're the cutting edge of, you know, memory or the cutting edge of other semiconductor processes.
And you see that in China as well. Increasingly, Chinese companies are investing in new R&D and they're the cutting edge of battery technology and, you know, electric cars and so on and so forth in India. These conglomerates, which have been around for a very long time, and also, you know, historically, predate many of, you know, the expansion of many of these East Asian businesses, right?
Like the Tata group has been around since Pre independence, it was huge pre independence, right? haven't invested in new technologies in the same way. and they haven't sort of done, they haven't done, this long term R&D that you would need in order to be at the forefront of some technology globally.
Right? And as a result of that, those companies largely sell domestically within the Indian market, right? Whereas these other conglomerates that I'm talking about in East Asia, they sell globally, right? They export their cars, they export their batteries, their semiconductors, and so forth. So I think that is, you know, one huge problem that we have in India, which is that these big companies that have the resources, billions of dollars in cash, don't invest in R&D, right?
And yes, I think that is a broader cultural problem. That's not specific just to those businesses. The government doesn't really invest in R&D in India. We invest a tiny, tiny fraction of our GDP, you know, compared to not just rich countries, but also developing country competitors. there's just, you know, fewer dollars available for whether it's bio R&D or other R&D, right?
And then I think there's beyond that an unwillingness even among venture capitalists, you know, who should be taking sort of technical risks in India to invest in businesses that are technically risky or to underwrite technical risk in part because they don't have experts that understand it. Right.
Almost no venture fund in India, like a, you know, sort of generalist venture fund has even like a biotech partner or biotech arm, for example, right, where they can evaluate biotech businesses. And that's just one example, right? There are all these other deep tech, hot tech businesses where they're sort of not really able to evaluate.
[00:06:21] Abhi: I remember you had one of the, like a crazy line and, like a document I read of yours where like some guy closely associated with the Serum Institute of India has invested more money into his own Bollywood production company than in any early stage R&D. Yeah,
[00:06:33] Soham: that's maybe a bit hyperbolic, but the guy who owns the Serum Institute of India that runs the Serum Institute of India has invested a bunch of money in, in, a Bollywood production company, like a hundred million dollars very recently.
Karan Johar's production company, he's a very famous director. and, they invest, yeah, not all that much money into early stage discovery R&D. So, you know, it may in fact be more. Is it like
[00:06:57] Abhi: And instinctively, is it fair to say, like, if he took that a hundred million dollars and said, put it into early stage R&D, it would be money well spent, or is there like some other fundamental problem that's going on where like money is not allocated.
Like even if the money is allocated, it will not be used particularly well.
[00:07:13] Soham: Within that company or within...
[00:07:15] Abhi: Within like, like all of R&D and in India,
[00:07:17] Soham: I think there's like more than a hundred million dollars of spare capacity, you know, in terms of things that could be done projects that are almost ready to go, that no one is able to find money for.
So I think, yes, I think a hundred million dollars in R&D would be extremely well spent and I think PopVax is a good example of this, right? We, have spent not. More than, I think, I think it's like, you know, on the order of 15 million dollars, right? Since, since we started three and a half years ago.
Plus minus. I think that investment would have been a good investment for many Indian pharmaceutical companies, right? And we have this Indian pharmaceutical business, right? Companies that export even to the U.S. Or export to Europe. But it's all generics and biosimilars, right? So they, they make copies of drugs that are either off patent or about to go off patent.
And they make them very cheap at high volume and, you know, in some cases very high quality. And this is good for public health and it's good for the world, but they don't invest in R&D. Right? R&D would be the next obvious step, one would think, right? And in East Asian countries, again, it has been the next step, right?
Companies start doing copycat stuff. They start doing generics or biosimilars in biotech. And then they wake up and they go like, oh, we could do, you know, we could do better. We could sort of do something that's, that's novel. And that's what you're seeing in Chinese biotech today, right? Companies haven't done that.
Lots of companies had the 15 million that PopVax has cost so far. Sure, they didn't have me or some of the talent that we have, and maybe they wouldn't have been able to attract exactly the same people, but maybe they would have been able to get people that were, you know, somewhat at the same level, and maybe they would have been able to do so even easier than I was, because I was a nobody who didn't know any biology, right?
But none of them did spend that money in practice, and so they didn't, you know, have the assets that we have and the platform that we have. Yeah.
[00:08:56] Abhi: I, like, like, like America has this America, like uniquely American trait of having like billionaires who are very interested in research and specifically biology research.
Like you have Jeff Bezos funding Altos labs. You have Patrick Collison funding Arc Institute and like Brian Armstrong funding NewLimit. Is there, like, any Indian billionaire who's, like, willing to, like, like, be in, like, play in that same space of funding, like, very high risk, high reward biology research?
And even if there is, do you think, like, their money would be actually, like, well spent in India versus, like, anywhere else?
[00:09:25] Soham: So, Kris Gopalakrishnan, who's one of the Infosys guys, has put a bunch of money into, into neuro research and brain research at IIT Madras, I think, and also in some institutes in Bangalore.
So I think he's maybe one good example of this. And then Azeem Premji, from Wipro runs, is sort of the person behind Azeem Premji University and they do some research and so on. But not, you know, anything close to the scale of what you see in the US. And also, I have talked to billionaires in India who should be interested in funding this kind of research, and without naming any specific names, they've been quite dismissive of the possibility that good research could be done in India at any price, and that's their excuse for not spending the money.
Like, the
[00:10:06] Abhi: Talent doesn't exist here, so why would they spend the money?
[00:10:09] Soham: Which
[00:10:09] Abhi: isn't true, right?
It's not like like, empirically,
[00:10:10] Soham: It's not. Empirically, it's not, right? And then on top of that, they I think, you know, they're sort of a... a minimum level of philanthropy that U.S. Billionaires engage in, even when they're criticized, they generally engage in like some minimum level that's like reasonably high, even if it's not for, you know, out of the goodness of their hearts, it's because they have interest, right?
Indian billionaires, especially ones who are multi generational, right, it's their father's money, or their father's father's money, or so on and so forth, right? I think tend to be uninteresting, and uninterested, and incurious, and so they're not fundamentally interested in funding your research. And they, you know, find convenient excuses not to do it, right?
I think fundamentally, there's a problem of cowardice in India, right? In our elites. Our elites talk a big game. Increasingly, they talk a big geopolitical game as well. But when it comes to actually doing difficult and risky things, we tend to shrink away from it, right? And so the business community in India has I've historically been very interested in finding ways to put up regulatory barriers to prevent access to the Indian market and ways for, you know, their monopoly or monopoly esque businesses to make more and more money off Indian consumers, and essentially no time on how to make globally competitive products that they can export to the rest of the world.
And this is, again, in direct contrast to what you see in many other countries. And I think it comes from the same fundamental problem that makes them also not interested in investing in R&D, you know, just at a fundamental level, they don't, this is not interesting to them, right? They would like to find a way not to have to do this hard thing if they really could avoid it, not have to think about it, right?
And so as long as there's, you know, sort of government support for enacting these barriers that will prevent, you know, foreign businesses from coming in and doing business in India, and they have enough of a market locally, even though I think from a strictly financial perspective, it obviously makes a ton of sense for them to sell stuff to bigger markets, right?
India is big, but like the U.S. is bigger, right? They just don't do it.
[00:12:18] Abhi: I mean, like you mentioned earlier, like, like the chaebols in Korea and like you see of this phenomenon also play out, like in China, Japan, Taiwan of like being very focused on manufacturing and then like pivoting to R&D once they get enough money.
Well, why do you think that didn't happen in India or like, do you think it's like underway and we're just like not seeing the fruits of it yet?
[00:12:36] Soham: Oh,
[00:12:36] Abhi: because they're
[00:12:37] Soham: cowards. Like, it's like, there's a, there's a, it is a cultural problem, right? Like, I think like the, the people, the same sorts of people, like the third, fourth generation Samsung people who are like, oh, let's invest.
Roughly speaking, like, all of our free cash flow into semiconductor, right, 20 years ago, the people in India who are sort of in the same positions did not do that, are not interested in doing that, aren't doing it now, right, for whatever the equivalent is. I'll give you an example of this. Nandan Nilakani, who's not a, a sort of a dynastic billionaire, but who's one of the Infosys billionaires, again, one of the other Infosys billionaires, gave the speech, maybe he said this multiple times.
But he said like, Oh, India shouldn't train its own foundational, like LLMs. So it shouldn't train its own foundation models. it's too expensive. We just shouldn't do it. And we should instead focus on specific applications, like making better call center chatbots.
[00:13:30] Abhi: That's bizarre. That's such a, that's such a like pessimistic view of the world.
Or pessimistic view of the nation that he's in.
[00:13:37] Soham: Exactly. And it's also like, we are a country that knows how to do cutting edge research for less money, but we shouldn't make a nonsensical virtue out of frugality, right? Sometimes you just got to invest the money, right? And DeepSeek just came out with their reasoning model, which they've made for like almost no money, it looks like, right?
[00:13:52] Abhi: And they also said this thing about like,
they were one of the, the, the CEO of whatever hedge fund is behind DeepSeek, like was asked, like, where'd you get the talent to build DeepSeek? And he said something along the lines of like, we did not brain drain like the U.S. We developed the talent in house.
[00:14:08] Soham: It's like a hedge fund, right? It's like a hedge fund that like decided to do
[00:14:11] Abhi: Yeah,
[00:14:11] Soham: so clearly they had smart people
[00:14:12] Abhi: to begin with. But like still, they didn't have access to like the OpenAI like employees or Anthropic employees. They developed it in house.
[00:14:18] Soham: It's like lots of prop funds and hedge funds have big offices in India.
Like Optiver has a huge office in like in Maharashtra, right? And I think in Bombay actually. Like you could totally pull some of the best talent in the country in if you paid them enough money to, build a, you know, new AI research shop, I think, right. If, if that's what you want it to do. And I think there's lots of areas where actually Indian scientists have made substantial strides in the past, right.
That were far ahead of where they should have been given the investment. Like we had really good nuclear reactors for less money. We had really good rockets, like all of this kind of stuff where if ISRO, which is the Indian Space Research Organization, which so pre SpaceX, ISRO had the lowest like per pound launch capacity, of anywhere in the planet, right?
So we should have been like global leaders in commercial space, and ISRO was just heavily under invested in. . So, you know, the program stagnated, and they never worked on reusable or sort of, you know, these, these sort of new innovations that would have reduced the cost of the launches further, and made it more frequent to be able to launch on.
They just got, you know, whooshed past by Elon. But there was no, like, fundamental reason. The rocket scientists were good, and the computational folks were good. Those two things could have been put together. It's just no one thought to do so. And now we have a commercial space industry that's flowering in India a little bit, right?
But it's so far behind what it could have been if people were willing to make those investments when there was still substantial. Like today, if you go, like, you need to make a reusable rocket that can sort of land, right? There's a proof of principle you can do that. And so it's a bit less technically risky.
I think the problem is, when people can't imagine, you know, something for the first time, right? When they haven't seen it actually done, and it's not a copycat, right? It's not just, we'll make it cheaper. The willingness and the wherewithal to underwrite that kind of technical risk, like first in the world products or first in the world R&D exists nowhere in India, right?
And it should, it absolutely should. We are one example. Many other people are, you know, I think shining examples of sort of, you know, even with less money, people are able to do this, but there's this sort of constant bottleneck on the ecosystem of like, you know, the people who have the money saying that there's not the talent right over and over and over again to convince themselves, I think that they're not just being lazy and sort of fearful.
[00:16:31] Abhi: And so there's like a huge bottleneck placed on the people who are actually like, like, courageous enough to think like this is possible. And then they just have barriers put in their way.
[00:16:38] Soham: And then they just leave, right?
[00:16:39] Abhi: Yeah, that's true.
[00:16:40] Soham: They just come to the U.S. And like, suddenly when you get on the plane and you reach here, you're in Boston or San Francisco and you're like, I'm going to do first in the world thing.
Even if it's the exact same team, now you can raise money suddenly, right? Which is unfortunate. And I think, you know, we've been, people, investors have told me, like, oh, your technology is really good. It's, you know, our preclinical data for influenza and for COVID is just about as good, if not better, than what anyone else has, right?
And people have been like, why don't you just move the whole kind of company to Boston, right? Like you're just so much easier to fund. If you, you know, it'd be easier for us to write the check if you just move the whole thing. And it's not because the talent that we have is bad today. It's just because of sort of, that's just what they understand.
[00:17:16] Abhi: Yeah.
[00:17:18] Soham: So I think if I'm a US investor and I don't fully understand that India has the talent, like I can excuse that to some degree. That's something we have to educate them about. If I'm, you know, a rich person in India or a powerful person in India, and I don't see around me the wonderful talent that is being crushed by the system, then I'm blind.
Right. And I think that's, you know, that's what's going on. Unfortunately.
[00:17:38] Advantages of building a company in India
---
[00:17:38] Abhi: On the topic of like why you incorporated in India to begin with instead of the United States, I think it's interesting to consider like the comparative advantages that certain places in the world have for certain types of research.
Like America has like Harvard's and MIT's of the world are able to create the craziest looking antibodies. China has incredibly low, like has access to like looser drug approval guidelines. I think it's like a guilty or like innocent until proven guilty drug approval and, so they can move a little bit faster, with that context, what are some advantages of operating in India specifically?
[00:18:10] Soham: So I think there's a lot of talent in India. it's not well recognized, I think, how much wonderful wet lab talent there is in India and how much wonderful computational talent there is in India. and. The cost of operations, is much lower. So, you know, 5x or 10x lower.
So we can do, we can hire people and we can do wet lab experiments and especially in vivo, like animal experiments. Thousands of animal experiments, and we've tested like a thousand plus novel vaccine constructs in vivo now. Much more cheaply, five or ten times more cheaply than you can in the U.S. or, or you can in, in, you know, in places that have similar economic structure, right? And so I think what that means is, if you look at, pharmaceutical program, right? These programs cost billions and billions of dollars, right? In some cases, a vaccine program, you know, could cost a billion dollars and still fail quite badly, right.
If we could reduce the cost structure of these programs, we could make it possible to test way more things up front then you would be able to basically then you would have the bandwidth to in a typical US pharma context. And then I think the other interesting thing that we can do in India is like what we've done is we have end to end you know all of our concept to clinical production in one roof, right?
And we are able to access relatively quickly talent for all those pieces, right? And so that means we can kind of jointly optimize end to end over the whole pipeline of things we're doing, right from concept to making it possible to produce, right? And that's something that big pharma companies can do in the U.S., but that small startups are often not able to do. And that means they often encounter translational bottlenecks in between. So for example, you know, I know companies, vaccine companies that have waited one, two years to get access to a CDMO, like a contract manufacturer, to be able to go into phase one in the first place.
And then that gap can kill the company, right? Someone will get ahead of you or you just run out of money. We have the ability, because it was relatively cheap to build that facility in India to do small scale manufacturing, to just, you know, be able to do that end to end, not be delayed by what external people are doing.
And, you know, the same thing for analytical operation, for animal house operation, you know, for, you know, for our teams working on, all kinds of, of, of assays that you might typically outsource in, you know, immunology assays or high throughput assays that you might outsource in a in a U.S. pharma context, right? And I think having all that under one roof, being able to scale that up relatively cheaply, and then just being able to hire more and more people, more hands, so to speak, quite reliably in a way that I think you just don't have access to that number of people that can do wet lab work necessarily in any, company in the U.S. because you have so much competition also with other innovative companies that are doing interesting works in the Boston area, right? Gives us the ability to just test way more things way faster be able to advance them into clinic more quickly than you would with the same structure, in a US company. So it's I think it's cost and just amount of talent. There's a number of people available who can do the wet lab work that then sort of you know compound into this broader advantage of being able to test more things
[00:21:11] Abhi: Like end to end.
[00:21:12] Soham: End to end.
Yeah, test them more quickly and then move them into clinic.
[00:21:14] Abhi: If you look at places like, like China or like like emerging biotech markets, like outside the United States, do you see kind of like the same parallel? I'm not super familiar with, drug developers in China. Do you, do you see like the similar, like,
[00:21:28] Soham: I think that's what they're doing in China.
Like,
so what I've heard is, and this is hearsay, but I think this is what is happening. Yeah. You probably heard that like this year it was like, or in 2024, I think 30 percent of assets licensed by big pharma were from China and was previously 50
[00:21:42] Abhi: percent of like oncologic INDs or something like that. And like
[00:21:45] Soham: Previously, it was zero, right?
Big pharma was not buying anything from China. I think what has happened is like some version of like, I'm a professor at Harvard and I read a paper and I'm like, I found this cool new cancer target. I'm going to make an antibody and I go to like, you know, Third Rock Ventures or whatever. And I get, I start a company to do it.
And I'm like, I announced my board of directors and my SAB and like, you know, I get some office space in Cambridge and like about a year has passed. And by this point, the Chinese company that read my paper, that read my preprint has an asset in clinic. Right. and I am screwed. And I think that is what it's like in China because they have the ability to, to effect that pipeline end to end, as we are trying to do as well.
You know, they can take a new target or a new idea and put it into their engine, right? Which is like unfashionable in the U S to have the whole engine right now. You, you have all of these like new, like labless ventures that are being spun up. They have the engine, they plug in the thing. And what I found is maybe this is just me as a non biologist, not being a good manager, but I think it's more general, right?
When you are in the lab with the people who are doing the work, you can spot issues in the data, or you can spot issues in protocol. If not me, my team can spot them and correct them in real time. And that compounds. So if you like go, go to WuXi and you're like, hey man, like do this assay for me. And then a month later you get the readout and you're like, oh, this is all trash.
Which happens, I think, quite frequently, right? That's a month lost, whereas I'm going to figure that out in two days, right? And so I'm going to correct, and I'm going to be suddenly now a month ahead of you, right? I think that's what's happening in China, where they, like, co located with their labs, right?
They understand, you know, in some sense, developability, right? Like, much more cleanly, because they know what's working and what's not, and they're able to, like, discard ideas or molecules that aren't working.
[00:23:25] Abhi: Like ballpark figure like if you had if PopVax had started in america, how much more of a multiple would you need to like run the exact same experiments we're running right now?
[00:23:34] Soham: I think it would have been like between 5 and 10x more money.
[00:23:36] Abhi: Okay.
[00:23:37] Soham: And like maybe now we have a lot of programs now and we're able to do them very very cheaply I think it would be. You know, instead of the company being like single digit million dollars per year to operate because we're 70 people, right?
We're 70, like mostly wet lab scientists, mostly folks with at least a master's, mostly with PhDs, right? And and you know, the requisite amount of, of, of sort of animal work. We do a lot more animal work than most vaccine companies. We have thousands and thousands of, constructs now that we've tested in vivo, and we've used, I think, probably 10,000 plus animals, actually more, more than that.
And so just, just the cage capacity, the animal handling, and sort of, you know, having the team alone I think would balloon the cost up 10x from where we are. And you can see that when we look at other vaccine companies, that, you know, are publicly disclosing their financials that, you know, went public or whatever.
You see that, which is at the sort of late preclinical stage, just before phase one, some of these companies are spending 50 to 100 million dollars a year.
[00:24:38] Abhi: Mm hmm. Yeah. And I think, like, going back, sorry, going back a little bit to, like, the cultural problems in India that have, like, prevented this sort of, like, high risk, high reward R&D work.
The UK is also going through a bit of this recognition of his research stagnation and has formed ARIA to combat it. and for like context for listeners, ARIA is a kind of a parallel to America's DARPA, so a funding vehicle for extremely high risk, high reward scientific or engineering projects. Is there like a similar initiative in India is like, I know like ISRO maybe was at some point.
Is there anything like that today?
[00:25:11] Soham: No, not that I'm aware of. So, they have recently reorganized the scientific funding structure of the various different scientific funding organizations that were in the country. I think they've rolled it into one organization, which they're calling the National Research Fund or the National Research Foundation.
But there is no, DARPA style agency or ARPA style agency, that has these kinds of specific program project mandates that they then execute. There is a, an organization in biology called the BIRAC, which funds sort of industry research. but it's really nothing like a DARPA or ARPA style organization at all.
It's really focused on present day incremental work and the funding amounts are very small.
[00:26:00] Abhi: I mean, that lends well to like the next question. Yeah.
[00:26:03] Policy prescriptions for India
---
[00:26:03] Abhi: What are your policy prescriptions to make India a global leader in biotech?
And maybe like, like R&D in general.
[00:26:11] Soham: So yeah, so the government should spend much more money on R&D. We should spend like as much as Israel does in terms of GDP percentage, like single digit percentage of the GDP at a minimum, like on R&D, right? I think this is the future of the Indian economy. We have to build products that no one else in the world can build.
Like clearly the world is becoming more protectionist, right? Just, you know, manufacturing something that everybody else can manufacture that maybe automation will take over in a few years is not going to be sufficient. We have to make things that nobody else has because that's when people are forced to purchase them, essentially, right?
And we have the talent, I believe, in India and Indian origin talent that we can import to do this, which brings me to my next policy prescription. So, are you familiar with the Thousand Talents program, the Chinese program?
[00:26:51] Abhi: Oh, yeah, like
bring, bring talent from the United States back to China. Exactly.
[00:26:55] Soham: And some of those people aren't even Chinese.
Like they're like just, I think some of those are just white people that just want to, you know, come to China for some arbitrary amount of money. So, we should have a Thousand Talents Program for India where we have India, especially Indian PIs, but also other people who are really good, who want to come back to India or want to come to India and work on what they're working on.
And we should give them a large amount of money over, you know, four or five years to do this and build labs, you know, in India to attract the best students globally to do this. And this is the ideal time to be doing this, because I don't know if you've noticed, but anti immigrant sentiment is building very quickly here in the U.S. and Canada and other places, right? In Canada, they've now cut, I think, student visas in half or more, right? So you're not going to be able to go to Canada as easily to do your PhD work, whether you're from India or from somewhere else, right? In the U.S. Who knows what's going to happen, but I don't think the environment is necessarily going to be conducive to more people whether it's starting here or sort of coming in here on H1Bs.
Maybe it will, maybe it won't be. So now is the time, if the Indian government is smart about it, to basically say, we're going to, be very open to scientific talent, Indian scientific talent, Indian origin scientific talent, but also scientific talent from all over the world that wants to work in a democratic, free country, which has a wonderful legacy of scientific research, come in and build, you know, your scientific legacy here in India.
And here's the money to do it. And so if I was in the Indian government, the Thousand Talents Program equivalent is the number one thing that I would do to change the trajectory of science research in India. I
[00:28:21] Abhi: mean, like the Thousand Talents, like the existence of the Thousand Talents Program sounds like an implicit recognition by the Chinese government that like a lot of talent from the country is going elsewhere to work in, work in areas that clearly don't exist in China.
[00:28:32] Soham: Which has happened in India.
[00:28:33] Abhi: Sure. Yeah,
like exactly. And like, it feels like, If, if China has explicitly recognized it, is there like some parallel to India, like recognizing that like graduates of like IITs and like talented people in general are just leaving India? Has there been any like national policy recognition by India that like attempts to stop the brain drain or do they just like not, not to attempt to engage with it?
[00:28:53] Soham: I'm not aware. So the government has not put up barriers, substantial barriers. They may. They may not. I worry that if they recognize it, it would be to try and just put up authoritarian barriers where they'd be like, you just can't leave now, which I think would suck. You know, you have the Singaporean version of this where, like, they have to do three years of, you know, You know, working in Singapore, like some years of working in Singapore, or something like that, even if you're from outside of Singapore, you know, if you, if you go get a degree at NUS with some money from the Singaporean government or whatever.
I, I think that, there has been no substantial recognition of this in a positive way, at least, like, no program has been set up to do this, which you would expect. You know, India's great advantage globally is that our, you know, immigrants from India are all over the world in extremely powerful positions in all fields, right?
And those people ideally could be used to, you know, form a core of an intellectual workforce that can help improve things for us, you know, in India, not to say that, you know, Indian faculty or folks doing research in India shouldn't be funded. They should also be funded substantially. But I think, you know, them having access to better colleagues and better students who would be attracted by researchers coming from top universities across the world and coming to India, I think would change the game, right?
But I haven't seen any movement yet towards anything that looks like this.
[00:30:13] Questions on vaccine design
---
[00:30:13] Abhi: Yeah.
Okay.
Hopefully it happens.
I think like outside of these, like those discussions of like, you know, Indian cultural problems and like policy recommendations, I have a lot of questions about like vaccine design in general. So you have antigens which are the molecular targets that vaccines train the immune system to recognize. But you also have immunogens which are what you actually put in the vaccine to generate that immune response, correct?
Sometimes they're the same thing. Sometimes they're not. And that's led to some really interesting approaches in vaccine design. Could you walk us through how our understanding of this relationship between antigens and immunogens have evolved since the birth of vaccinology?
[00:30:49] Soham: Yeah. So, I think the, the critical distinction between an immunogen is that the immunogen is sort of what goes in and elicits the response that you want. And I'm going to talk really in the context of antibodies, because that's what we work on at PopVax, and I think that's sort of simpler than talking about T cells.
But basically, the immunogen in some sense is what goes in, that elicits the antibodies that you want, that then, you know, whether in the short term or in the long term, form the core of the immune response against the actual pathogen, which has the antigens, right? And I think what's critical to understand is, you can have, you know, an antigen that is on the pathogen, right?
That isn't necessarily the right immunogen to use in your vaccine, right? Or you can have sort of a native form of the antigen that is on the pathogen that, when modified, would be the right immunogen, but when you put it in its native form, either doesn't elicit the antibody response you want, or the immune response you want, or even worse, causes problems, right?
And a good example of this is in, in RSV and respiratory syntactical virus where like, there's the RSV fusion protein or F protein. That's what's used in a lot of RSV vaccines as kind of the, the main, antigen, from which then immunogens are designed. And it turns out when you introduce the RSVF protein in its native form, right, when you in some sense take the antigen as it is, right, and you use that as your immunogen, it elicits antibodies that actually for people who have not yet been exposed to RSV, cause antibody dependent enhancement (ADE) of the disease, where it elicits antibodies that which, you know, bind to the pathogen in some way, but actually inhibit the ability of your immune response to clear the pathogen, right, whether that's inhibiting other antibodies or, or sort of in other ways, right?
And
[00:32:46] Abhi: Is that, is that just because like the antibodies generated by the RSV immunogen are just like not good enough?
[00:32:53] Soham: So typically, when you see ADE, you'll see something like the antibody binds, it binds really tightly, but it doesn't neutralize. So it doesn't prevent the you know, your, your virus from entering the cells.
And what it actually does is it prevents other, better, neutralizing antibodies from binding and preventing. Right, and so it like, it pulls away the surface that they could use to stop the pathogen, but it itself doesn't stop the pathogen. Or they're very weakly neutralizing. Right, and if you have overproduction of these antibodies, because that's the response that the body remembers to make.
Right, You know, memory B cells, you know, of this particular type of antibody, then you crowd out better antibodies that might actually be able to neutralize or stop the pathogen, right? and so in the case of the RSV fusion protein, the antigen is the RSV fusion protein because, you know, the antigen is what's on the pathogen.
But the immunogen that works, now we have several approved RSV vaccines, is a modified version of this, where you lock it in its pre fusion conformation. by doing two mutations, or a few mutations, rather. and it's similar to the mutations that have been made in the spike protein for COVID 19 that were used in those vaccines, right?
those were the S2P mutations. and actually invented by the same guy, Jason McClellan, right? who's a structural biologist who's pioneered this work, where basically he figured out, and this is kind of intuitive, if you have your, protein that, that binds with the cell receptor. So it's a cell entry protein for, a virus.
And there's a whole class of these called class one fusion proteins. And, you know, RSV is a class one fusion protein and COVID is a class one fusion protein and so on. Right. they exist in a pre fusion and a post fusion conformation. So they're in a, there's one shape that they take before they bind with the cell, right, before they bind with the receptor, and there's another shape they take after they've already bound with and the pathogen has entered the cell.
Right. That second conformation is kind of useless to elicit antibodies, because it's already
[00:34:41] Abhi: inside. It's already in,
[00:34:42] Soham: yeah. So what you want is you want to lock the protein in the conformation, that it, you know, that it is before it binds, so that you get antibodies to that conformation that may be prevented from binding, either by directly blocking binding with a receptor or by blocking it from changing shape.
Right? but the pathogen is crafty, and so it's not going to show you that conformation natively a lot. And so, pathogens where that is the case, if you show the native version, you typically get bad responses. And in this case, in the case of RSV, actively harmful responses. And so here, the immunogen that you want to use is the RSVF protein in this particular conformation, right?
but the antigen natively is not in that conformation all the time, or most of the time, and so it doesn't give you the antibodies you want.
[00:35:26] Abhi: And typically this is something like people only discover after like structural characterization.
[00:35:30] Soham: Well, in this case, people discovered it because they injected RSV vaccines into people.
It's just like, okay, it
[00:35:35] Abhi: didn't work.
[00:35:36] Soham: No, it, it made it worse. And so that's why, like, I think this was in the eighties, like a bunch of like RSV vaccine trials and then it all went horribly wrong. and then we just didn't have RSV vaccines until now, like in the last two years, there've been new RSV vaccines approved for the first time.
And all of them use this prefusion conformation version of the RcFusion protein.
[00:35:59] Abhi: Does a similar problem exist with like, like, like the COVID 19 virus as well?
[00:36:04] Soham: So all of the vaccines that I'm aware of use this prefusion conformation. that, that are recombinant, that use only the spike antigen. There's also, the, there's also the inactivated vaccine from Bharat Biotech in India that I don't think uses this, to my knowledge.
And it still kind of works, but it's not as good. So I think in COVID, it doesn't actually cause ADE. So COVID doesn't seem to have this ADE problem, but it just makes it better. And it produces a sort of more neutralizing response to lock it into this conformation.
[00:36:36] Abhi: That feels like, the.
The realization that there are different conformations you need to be aware of when designing immunogens feels like, like a, like a step level change in your ability to design good immunogens in the first place. Is there like another one on the horizon that you see that like, in terms of like, like a new bit of vaccinology that we're stumbling across that leads us to design better immunogens, or is it kind of like fuzzy right now?
[00:37:00] Soham: So I, yeah. Okay. So to go back to this antigen immunogen difference and then answer your question, right? So an antigen is anything. you know, that that sort of binds to an antibody, right? That's that's what the definition is, right? And then an immunogen is something that elicits, in the case of antibodies, that, that antibody, right?
So the immunogen for that antibody is the immunogen that would hypothetically elicit that antibody, right? I think what's changing now in vaccine design and what we work on a lot at PopVax is an extension of, of what we've just talked about with RSV, where you really want to elicit specific types of antibodies, right?
That do specific things, that have specific functionality. They, you want them to neutralize, right? You want them to neutralize broadly, right? Right? so, in the case of hepatitis C, which is one of the pathogens we work on, the pathogen mutates in the host. And so it presents as a quasi species, which means that If you try to knock down just one, you know, iteration of the quasi species, right, one particular version of the pathogen, maybe that's not sufficient because there are all these different mutated versions in the body, right?
So you need to, to get rid of hep C, you probably need, you know, broadly neutralizing antibodies. Antibodies that are able to go up against a whole bunch of mutated versions of the pathogen. and so I think what's changing in vaccinology now, is we're asking the question for the first time, can we elicit specific classes of antibodies, right?
that do these specific things and not other antibodies that are bad, right? So we find like a set of antibodies to elicit target antibodies. And increasingly, next generation sequencing techniques, like, you know, for example, doing single cell B cell sequencing allows us to go and identify specific antibody clones, right?
Like specific B cells that have a specific antibody. in patients who have been able to clear the pathogen or have had good outcomes, right? And then check the functionality. Is it broad neutralizing? You know, does it have a effector function? Is it is it good in some way and then try to actually elicit them, right?
And this is something that started in HIV vaccinology because there are these people in HIV called elite controllers who are able to control the progression of the of the disease. It doesn't go to, you know, it doesn't go from HIV to AIDS, without having antivirals, right?
[00:39:10] Abhi: Just like innately.
[00:39:11] Soham: They're like, they're just able to do this, right?
And so it was believed for a long time that, in fact, maybe true, that they are able to elicit these. you know, particular classes of, of broadly neutralizing very potent HIV antibodies. and at least in some cases for elite controllers, that does seem to be the reason, right? But they're a tiny, tiny fraction of the population.
And for a long time, HIV vaccinologists were like, let's try to get people to elicit these specific antibodies, right? And then we'll have a vaccine for HIV. The problem is these are extremely somatically hypermutated. So they're like 8, 10, 12, whatever, mutations away from the germline, naive version of the B cell to get to this antibody.
And it's a very torturous path to be able to elicit them. But the other problem is like fundamentally they believed, oh, if I could have an immunogen that binds really well, right? to this antibody, then I can elicit that antibody, right? And so they did a lot of like yeast display and stuff to try and find immunogens, like versions of the epitope, which is this like specific segment that is immunogenic and elicits the antibody, right?
Or elicits the immune response. they're like, they've tried various ways to, you know, you know, to get these epitopes to bind better with their antibody of interest and they're like, oh, and then when we put this in humans, the best binding one is most likely to be able to elicit the antibody. As it turns out, very expensively, we found out that's not true, right?
It turns out that binding is not a sort of complete representation of elicitation. In fact, they're quite different processes, right?
[00:40:35] Abhi: Sorry, going back quickly, going back to the HIV thing. Yeah. I still don't quite understand, like, why, why. Why can't we just like replicate this specific antibody, like the ability to, like an immunogen that causes this specific type of antibody to appear.
Why can't we just, why can't we just do that?
[00:40:51] Soham: Well, we don't know what it is.
[00:40:52] Abhi: Oh, like, it's just like, why don't we know what it is? Well,
[00:40:57] Soham: in HIV's case in particular, HIV mutates on the host too. That's true.
[00:41:00] Abhi: Okay, so like, even
if you could replicate it, like, you have this group of people who can resist the progression to AIDS really well, but each of the antibodies they develop that resist that progression are kind of unique from person to person.
[00:41:10] Soham: They're not, some of them aren't, some of them seem to be more broadly applicable. Okay. But it's, what you're hitting at is a very important problem, It's sort of not intuitive to understand, which is just because we have the antibody, we, most of the time, we don't know what elicited it.
[00:41:25] Abhi: Gotcha.
[00:41:25] Soham: Right? Especially in these pathogens where there's a lot of mutation going on, right?
and we don't specifically know what the immune system saw. What shape or conformation of the antigen that it saw from the pathogen that allowed it to elicit that antibody and sometimes maybe you just get lucky right and you like you see some cryptic pocket that's not usually exposed, but in this case in this patient it was and some B cells saw it and it really worked well. So that B cell replicated and then you got this right but it happens in so few cases that it's not a repeatable process.
[00:41:57] Abhi: I'm, I'm curious, has it been like elucidated, like what's unique about the, is there anything unique about these people or, is it just like they, they got lucky?
[00:42:02] Soham: There are lots of different hypotheses and I don't, I'm not an HIV expert, but I think it's like, it's a very hard field because there are lots of things that I think are simultaneously true.
And there's no one specific answer to the question of, like, what makes these elite controllers elite controllers. Yeah. And so what happened is basically, we, is, I think this exact thing, which was like, oh, we have the antibody, now it should be easy. And then, like, 20 years later, no HIV vaccine, it's not that easy, right?
[00:42:30] Abhi: Yeah.
[00:42:31] Soham: And in part because it was this dogma became like sort of, oh, if we make it bind really well, it'll elicit the antibody. And I think the answer is that's not true, right? And so we have to explicitly in some sense, you know, model the elicitation process. And so what we're trying to do at PopVax, and I think what is kind of the future of vaccinology as a field is, I have a set of antibodies that I want to elicit.
I need to be able to build a machine learning model or build some kind of, some kind of model one way or the other, right, that allows me to go from that set of antibodies to the immunogen that then elicits them, right? And the only way to do that is to collect a bunch of data about how libraries of differently designed immunogens elicit antibodies, right?
And go front and back and then use that as a way to actually design your immunogens.
[00:43:13] Abhi: Yeah, I mean, like, instinctively, like, before this conversation, I would have assumed, like, immunogen equals, like, something that, like, can, like, that binds really well. Yeah, yeah.
[00:43:21] Soham: But that's the antigen, right? And,
[00:43:23] Abhi: like, I, I guess.
The the part that i've been a little bit confused about like we've talked a little bit about like what is the what's like chemically going on that causes the elicitation process? Is that not well understood?
[00:43:34] Soham: It's it's I think it's sort of mechanistically understood to some degree. But there's so many different sort of there are different things that can happen in that process, so many different combinations of the of your You know CDRs or your variable reasons in the antibody that It's, it's not something that can be, I think, explicitly modeled from theory alone, right?
It's also, so if you want to go really wild about it, have you heard of Jerne's network theory?
[00:43:57] Abhi: I have not, no.
[00:43:57] Soham: Okay, so Jerne, and it, it, Jerne's network theory, by the way, is totally true, despite the fact that it sounds insane. So you have these cascades of immune responses. So I have my immunogen, it goes into the, the body, it elicits, like, it goes, you know, binds with some B cell, and then maybe there's some mutation and the B cell replicates.
It's closer to binding now with this antibody after the mutation. it turns out those antibodies can also elicit antibodies. Okay, like there's like
[00:44:21] Abhi: cross immune, like antibodies can talk to other antibodies. No,
[00:44:24] Soham: antibodies can elicit other antibodies. So the antibody goes and binds with a naive B cell, it elicits another antibody against the antibody, right?
and then those antibodies can elicit further antibodies. And then, but those antibodies, right, sort of the anti idiotypic antibodies they're called, are now mimics, structural mimics, of the, original immunogen. So you can do things, crazy things, like you can inject an animal, and you can go seek out these anti or idiotypic antibodies, and you can use them as an immunogen to elicit the immune response that you want.
[00:44:59] Abhi: That's bizarre.
[00:45:00] Soham: Right? And so it's a net, it's like this cascade of this elicits that, elicits this, and it's not just happening in one dimension because a single immunogen is going to list a whole bunch of different antibodies, right? And so it's, and then they're all, there's all this crosstalk going on, and somewhere at the end of that is my mature antibody that I want.
[00:45:17] Abhi: That actually like, like, neutralizes whatever pathogen you have. Okay.
[00:45:22] Soham: So it's, it's, it's not, it's not obvious to me that there's like a simple mechanistic way to simulate this out.
[00:45:28] What does PopVax do?
---
[00:45:28] Abhi: I haven't actually given an opportunity for you to like give the full overview of what PopVax is and like how you guys function. So feel free to just give me an overview.
[00:45:36] Soham: Yeah, sounds good. So Popvax works on broadly protective vaccines. We develop broadly protective vaccines, both against pathogens where there are existing vaccines, but we think they're not broad enough. So an example of this is COVID, whereas I've talked about a few times now, you know, existing vaccines didn't cover a bunch of the new variants that emerged pretty rapidly.
Influenza, which is another key indication for us where, you know, there, there are these seasonal vaccines that people take each year, but oftentimes those vaccines, aren't as effective as they could be because eight months ago, the prediction of what strain was going to be dominant was incorrect, or that strain has since mutated in such a way that it's no longer effective.
And of course, these seasonal vaccines don't offer protection against potential pandemic influenza like H5N1, which is now spreading in cows and other animals in the US, right? We also work on vaccines against, pathogens where there are no existing vaccines. An example of this is Hep C, which I mentioned, which is also sort of a broadly protective case, where, you know, there, as I said, it mutates a lot in the, in the human host.
And so you end up having to protect against not just one, pathogen, but like a whole quasi species of this pathogen to get an effective vaccine. And also, you know, for, for other pathogens like strep A, where, existing vaccine design approaches created some kind of, issue. So we've talked about antibody dependent enhancement.
In the case of strep A, existing strep A vaccines, you know, a few decades ago, caused autoimmunity because there was an antigen that they were using, in their vaccine that, that actually elicitated antibodies against a human protein, right? So, all of these are, pathogens where some kind of precision approach is needed, right?
Whether you're getting, you know, trying to elicit antibodies for broader protection or antibodies that are avoiding some kind of, response that would be bad with antibody dependent enhancement or it's, you know, it's some kind of autoimmunity. Right? And the way we're organized essentially is we do all of this stuff in house.
So we have our own mRNA platform that is able to, display immunogens in this repeating form on things that are similar to virus like particles that's encoded in RNA. So basically what you inject is a standard mRNA lipid nanoparticle without having to do fancy manufacturing, right? We design our own lipids for lipid nanoparticles.
So our own novel ionizable lipids. We have a library of about 400 of these now that that our formulation and delivery teams have worked on and we've tested now thousands of different lipid nanoparticle formulations, you know, many in vitro and, and, and even hundreds in vivo, that allow us to be able to basically pinpoint what the right lipid that we've designed and what the right formulation is to deliver these mRNA that encode these vaccine immunogens, And we've taken a very similar empirical approach to optimizing these as we have our immunogen designs, which I've talked about.
And then, of course, we design our immunogens, which we've been talking about quite a bit. But basically, we use, you know, precision immunogen design, to try and elicit specific classes of antibodies we're interested in, avoid antibodies that we're not interested in, or that, you know, could cause, you know, cause problems, in particular for, for broad protection that is also potent at the same time.
And, and we use this mRNA encoded, you know, virus like particle structure to display these immunogens in a way that massively increases the magnitude of the antibody response elicited and also gives us some additional breadth in terms of the VLP structure also helps with breadth. So we are about 70 people, based in our Hyderabad facility.
And we actually go all the way up to GMP production. So we, we make, clinical doses, we're making them for our first, COVID 19 phase one trial. And so we can really go from concept all the way to clinical dose production in this one facility with our, you know, end to end team. And I think that's also quite different from, from the way a lot of biotech startups operate in the U S today, where they contract out a lot of different work, exactly.
Whereas we kind of do all of this in house.
[00:49:27] Abhi: Like how, like PopVax does precision immunogen design, I think it's good to give something background context on how it actually works. So you have have like mRNA, in a, a lipid nanoparticle that encodes for a virus-like particle (VLP) that has a linker that is then attached to the immunogen, right?
So you have this like, like shield of immunogens wrapped around the, the virus-like particle. Does, do you use machine learning at every step of the design of the Virus-Like Particle, the linker? And the immunogen, do you focus on one over the other? Yeah, like walk me through how, like, a workflow goes.
[00:49:57] Soham: Yeah, so for our COVID vaccine, we have, this sort of self assembling protein, that we attach via a design linker to our immunogen of interest, which we also sort of, you know, redesigned to be more compatible with that. and, those, you know, basically together form a single protein that we encode in mRNA.
And that is, when it goes in the cell, it translates into this, you know, single protein all linked together. And then the self assembling proteins, you know, sort of join together, they self assemble. And then what you get is sort of something that looks like a virus like particle that displays the immunogen repeatedly.
We started by not using a whole ton of machine learning in this process. We used existing known self assembling protein. We used existing known linkers. We used, you know, sort of more manual sort of Rosetta based modifications to the, the immunogen, to try and present it better on this, this sort of VLP like structure.
And we just did a bunch of combinatorial optimization in vivo of what worked best, right? And so the advantage was, we just did a whole bunch of testing and we were willing to try, I think, as I was saying earlier, like a whole bunch of things and see what worked best, weren't really too wedded to what would work best with, you know, what we got is what worked best.
and, and then that, we,
[00:51:14] Abhi: Sorry, with the primary measure of efficacy being neutralizing antibodies?
[00:51:18] Soham: In this case, the primary measure of efficacy being two things. One is sort of neutralization magnitude, sort of, depth where we, like, how much better is it in neutralizing than the existing BioNTech Moderna vaccine sequences, which we use as comparators, right?
And so our vaccine, for wild type, when we did all these optimizations, and displayed it in this way, turned out to be, like 55X better than just the RBD immunogen at the same mRNA dose in terms of neutralizing antibody data against wild type. And 22x better compared to, the BioNTech sequence, basically sort of the existing wild type mRNA spike COVID vaccine.
And, and that was, you know, our, with our own experiments, you know, in vivo. So we didn't have the whole, the actual BioNTech vaccine, but we made the same sequence and we encapsulated in the same lipid nanoparticle so forth. Right. So a reasonable facsimile. And so that was one measure. And the other measure was breadth.
So are we able to, you know, as I said at the beginning, we were funded to do this vaccine, and I started the company, because, the existing COVID vaccines were failing as soon as there was a sort of new variant, right? And in India, we had the emergence of this Delta variant, which emerged in Maharashtra, where I was in Pune, where I'd moved my software for a robotics company, right?
And there was no real attempt by the Indian vaccine companies to make a Delta specific variant anytime. That would have helped, right? So we had a lot of people essentially die as a result of that. And so a big goal for me was, can we make a vaccine that has broader protection against variants? So the other big measure for us was, we had a whole bunch of variant pseudoviruses, where we had both existing real variants that had emerged, and we mutated these pseudoviruses to, you know, present versions of the spike protein that didn't exist in nature, but could very well, right?
And some of this was based on a data set from a guy called Jesse Bloom, who's at, Fred Hutch, who does deep mutational scanning, which I'm sure you're familiar with, right? So, like, basically, you know, mutate the, you know,
[00:53:10] Abhi: The single and double substitutions
[00:53:13] Soham: Yeah. Exactly. Exactly. All singles and then a couple of doubles.
Like some distribution of doubles, not all. Where, you know, it's again, a pseudo virus system where he mutates the spike protein. And then he figures out which of these are still stable and functional and then which of these are now able to escape, you know, antibodies that were elicited to the original vaccines.
And so we use that, not deep mutational scanning, but we sort of used, you know, versions of the spike protein from his assays that had shown this kind of escape potential. Okay. In our pseudoviruses, so not the live virus, so very safe, right, they are not functional virions. But, we use those as a, as a measure of how much breadth we're able to get.
[00:53:53] Abhi: You have these like two measures of efficacy of whatever you're designing.
And you mentioned that, when you first started off, you started off with like well known scaffolds, well known linkers, well known immunogens. Like where do you, where do you go from there on that?
[00:54:06] Soham: So from there, now what we're doing is we're using different, sort of self assembling proteins, some of which are de novo generated by our team.
We're using different linkers, some of which are de novo generated by, by machine learning. and then we're using versions of the immunogen, which are, in the way that I was describing before these precision immunogens where we either eliminate epitopes and then fill that in using machine learning or we are scaffolding only a single epitope that's intended to elicit a single type of antibody but we're still able to use this mRNA encoded VLP structure to substantially boost the neutralizing antibody response.
And this is particularly important for epitopes, which might be naturally subdominant, right? So, there's this notion of immunodominance. If you have a whole bunch of antigens in a pathogen, some of them are going to elicit a big response, and some of them less so. And some of that is due to T cell help, and there are other reasons for it.
But, let's just accept that as fact, right? Sometimes the best antibodies are elicited by epitopes which are not immunodominant, which sucks for you as a vaccine designer, you know, if you, if you're trying to sort of elicit specifically to that. So one way to deal with that is you eliminate the other immunodominant epitopes that you don't want, right?
And so we just don't show those, right, if we don't want to list antibodies to them. The other way is using something like what we're doing, which is this sort of VLP approach, which boosts the immune response even to these subdominant epitopes.
[00:55:24] Abhi: Gotcha.
Right. Is it, is it immediately clear like, which part of this, like three step redesigning or like, like three areas to redesign?
Is it clear like which one has the highest impact? Is it clearly the immunogen or is like the linker and the scaffold actually.
[00:55:38] Soham: So the immunogen gives you specificity. Okay, so like by redesigning the immunogen to make it better and better. We're able to get closer to eliciting the specific kinds of antibodies we want. By changing the linker and the self assembling protein, we're able to get basically a bigger magnitude of response.
[00:55:53] Abhi: Gotcha.
All right. Yeah, that makes sense. Historically, it sounds like, like, the initial step is, like, redesigning the immunogen, and then, like, once you've got a good immunogen, you start working on everything else. Or is it, like, not as cleanly segmented?
[00:56:09] Soham: No, it's not segmented like that at all. So, like, we do everything in parallel, basically.
[00:56:11] Abhi: Okay. Okay.
[00:56:13] Soham: Because we initially didn't redesign the immunogen that much at all. We started with this new, the presentation methodology. And then we started optimizing the immunogen.
[00:56:20] The role of machine learning in vaccine design
---
[00:56:20] Abhi: On the topic of like how you actually do this in practice, like PopVax is a biotech company, but also they're like, you guys have like a machine learning team and you're trying to use these protein foundation models for the purposes of, immunogen design.
Could you walk me through how like useful are these? Like, well, it's like AlphaFold, RFdiffusion. Are they revolutionary, somewhat useful or not useful at all?
[00:56:41] Soham: So, so we use, I think the way to describe what we do at PopVax is basically, we take existing protein design models. So as a diffusion models or protein language models that can spit out new proteins, that we basically condition on the task of scaffolding epitopes, which are, you know, the pieces of your antigen that, that, you know, that are specifically binding to the antibody, that therefore, you know, in some fashion, elicit the antibody, right?
And we use them to generate libraries of these scaffolds, that we can then test either in vivo, so in mouse models, or in organoid models, which are based on human cells, to see what antibodies they elicit, right, and then use that to inform a feedback loop where basically we can rank these immunogens, these design immunogens, based on how close they got to eliciting the antibodies we wanted to elicit.
and then use that, using RL or DPO or, you know, basically alignment and fine tuning methods like this to make the design models spit out things that are closer to what we want, that are closer to being able to elicit the antibodies that we want. Right.
[00:57:48] Abhi: Gotcha.
[00:57:49] Soham: So like we, as a base case, we use the existing models.
We don't train our own models on PDB, at least not yet. Right. but then we're using this, this dataset, this new dataset that we have basically, where we map elicited antibodies for the first time, which no one else is really doing to create a feedback loop that is able to get us closer and closer to eliciting the antibodies we want to elicit, right?
And so that's how we use these models. And I think in that sense, the models are very good at spitting out proteins that, you know, have stable structure and that, you know, we can thus use as, as test cases. and I think the models are amenable to being fine tuned and to being aligned. And so we're not the only person doing this in some context to the other.
People are doing this for antibodies. People are doing this for, you know, better performing enzymes, but data sets with hundreds or, you know, certainly thousands of data points can actually be used to move these models towards giving you structures that are closer to what you want. Right. and so I think in that sense that are useful and we've seen that they're useful in this way.
Structure prediction models, we use a number of those. Basically, once we generate these designs from these, from these models. and when we use sort of more manual methods of design, like Rosetta, we use the structure models to predict whether we're getting something close to what we want it to get.
Right. And we can use multiple of these different structure prediction models, as sort of orthogonal measures a little bit. They're not that orthogonal cause they're all trained on pretty similar data sets, but like somewhat orthogonal measures of like, you know, these two models are agreeing. Probably this is a protein that I would want to produce.
Whereas, you know, when the prediction is completely different from what the design model was designing in terms of structure, then we typically discard. So we use that as part of our down selection pipeline in silico.
[00:59:22] Abhi: Is it, when it comes to, like, machine learning actually used for this, is it clear, like whether like structure models are really good at this, language models are even better ?
[00:59:30] Soham: We mostly use structure based diffusion models. PLMs we've had sort of less good results with, but maybe that'll change. I mean, I think that there are new models that people are dropping, you know, very frequently. I think what's nice about what we're doing is we can use, other people's quote unquote sort of protein design foundation models.
We can fine tune them using a kind of straightforward, you know, set of methodologies and we can sort of use what's best or, you know, depending on what the licensing terms and so on and so forth are these models. In future we may, you know, develop our own sort of immunology foundation models, but I think we'll need a much larger scale of data than we currently have to do that.
[01:00:07] Abhi: Yeah. I mean, I'm not sure how much like information you're able to share about this, but like the actual design process, like. Like given like AlphaFold2, how do you use AlphaFold2 to redesign the immunogen?
[01:00:16] Soham: So like AlphaFold2 we use as a, you know, as a, as a kind of checkpoint where basically if we're designing, a protein that's intended to be in a certain structure, we use AlphaFold2 to predict out the sequence once we've generated the sequence, right?
So, if you're using a, a structure model that spits out a backbone, the backbone, then goes into what people call a, inverse folding model or like, you know, something like protein MPNN. Yeah, exactly. yeah. Some of these models are, you know, give you a sequence as well, right? And then we, we can predict out basically using Alphafold2, using some structural prediction model.
Is it conforming to the structure that we expected? And that if, if it's not, right, we, if we generate tens of thousands of options, we'll eliminate the ones that don't fold in the way that we would expect.
[01:01:00] Abhi: So I bet, I guess, like, how do you, like using the structure prediction model, what are you actually trying to optimize the end result for?
[01:01:08] Soham: So the structure prediction model, again, we're basically just trying to eliminate bad designs that we don't want to test in vivo.
[01:01:14] Abhi: Okay, and like how do you define a bad design ?
Like low pLDDT?
[01:01:18] Soham: Yeah low pLDDT is one example,.
[01:01:21] Abhi: So there's like
in silico metrics you're using to decide.
[01:01:24] Soham: Yeah. So we start with, you know, tens of thousands of designs and then we downselect down to hundreds that we would actually test in vivo. Yeah.
[01:01:30] Abhi: And so then like how do you take into account, like the data that you've tested in vivo, how does that get like fed back into this like redesign process?
[01:01:38] Soham: So we can create a preference ranking basically, right? So you got a set of antibodies, want to elicit, which of the immunogens got closest to eliciting the antibodies we want to elicit, and we can do this with function, which we were talking about earlier. So like, which of them, you know, neutralize the best, which of them neutralized the most breadth, right?
And then you can create rankings and then you can use different versions of these rankings to do DPO or do fine tuning basically.
[01:01:58] Abhi: I'm curious. Has it like, have you like, have you tried Boltz-1? Has it been like a big step up from AlphaFold 2?
[01:02:04] Soham: I, I'm not sure that we've tried it extensively enough to have an answer to that question.
Gotcha. Okay. Okay.
Structure prediction is not such a big bottleneck
[01:02:15] Abhi: So what actually is the bottleneck then?
[01:02:17] Soham: Yeah, I think we, it'd be nice to have... so the, the design models themselves are not necessarily so great at scaffolding the epitopes that we want. It's, they're still not able to produce the structures that we want and the conformations that we want reliably for some of the problems.
And so I think making those better and we're, we're, you know, we're doing our best also to try and fine tune these models to get better at those problems, even in silico before we get to the in vitro and vivo data, right, is more critical for us, whereas I think certainly structural prediction models aren't ideal.
But for the size of proteins that we're using, you know, we don't think that's sort of a big bottleneck to us being able to test these designs, in vitro and vivo, because even if we throw away some designs that are, you know, maybe actually good, things that are predicted with high confidence of these models tend to actually be what, what you would expect them to be.
[01:03:07] Abhi: Is it typically clear, like at the end of these, like design sessions, is it, do you have a good sense of like, what do you want the end antibody to resemble?
[01:03:17] Soham: Say again, please.
[01:03:17] Abhi: Like, you're, you're, you're designing an immunogen, you want to elicit some antibody response. Yeah. And you want this antibody response to be like good for whatever you're trying to protect against.
[01:03:28] Soham: Yeah.
[01:03:30] Abhi: Do you have like a gold standard antibody that you're comparing it against or are you purely looking at the immune response of whatever you've injected this into?
[01:03:38] Soham: So we can do both, right? So, and that's a good question. So, we can look at the functional response, right? Which is to say we can, inject into mice, any specific design.
We take the serum out of the mouse and then we can use that serum in a neutralization assay, in an effector function assay to see the functionality of the antibodies elicited. Are they able to prevent the pathogen from entering the cell? Are they able to clear infected cells? Right? So that is one thing, but we also have, say in the case of Hep C, there are these antibodies, which are clearance associated antibodies, sequenced out of people who are able to clear the pathogen.
And, and it's an important distinction here between HIV and Hep C, because in Hep C, it's like 30 plus percent of people clear the pathogen straight away. So it's not rare, right, compared to HIV, where it's like some fraction of a percentage.
[01:04:24] Abhi: So there's like some similarity in antibodies amongst 30 percent.
[01:04:29] Soham: That remains to be seen, people, I mean, lots of people have done sequencing at a small scale, not a ton of people have done it at a large scale in a diverse dataset, which is one thing we're trying to fix, but hypothetically, you know, you, you can get out a set of target antibodies, and we do have a set, some, a small set, at least, of target antibodies that we're quite sure will help clear the pathogen, based on that research that other people have done, right?
For those antibodies, we actually can do sequencing of the B cells that are elicited, right? and we can figure out how close we got in terms of sequence and predicted structure to those antibodies. Now, in a mouse model, those aren't going to be exact, right? but in an organoid model, we can get closer to what would actually be elicited in a human.
[01:05:08] Abhi: Is it like an insane idea to like, like hallucinate an antibody that binds to some like virus and then work backwards from the hallucinated antibody to design the immunogen that would cause that hallucinated antibody to exist. Does that does that at all make sense?
[01:05:25] Soham: It's not crazy. I just, I think the, the, I don't think you can do that without a new data set, right?
Because there's this sort of black box of elicitation, which is very complex, right? Even though it has simple constituent components because of, you know, all of this interaction, it becomes non trivial, right? and I think we don't currently have a dataset that allows you to, to, to make that jump, right?
People are doing de novo, you know, kind of hallucination design of antibodies intended to neutralize .... there was a paper recently on neutralizing, snake venom, right? You know, neutralize viruses. Like, I think there are ways to do that with existing datasets. Immunogen design in particular, I think, is a problem that cannot be tackled with these existing datasets in and of itself, right?
[01:06:11] Abhi: Do you think it's just like a scale question? Like, people haven't collected enough data yet?
[01:06:15] Soham: People haven't collected any data.
[01:06:16] Abhi: Oh, okay.
[01:06:18] Soham: I think, if you ask me, like, who has data that maps from immunogen design to what antibodies were elicited, I think the answer is me.
[01:06:29] The (conservative) culture of vaccinology
---
[01:06:29] Abhi: I mean,
I guess that kind of kind of raises a question.
Why, why don't vaccine companies do more precision immunogen design, like using these sorts of models?
[01:06:38] Soham: I'm glad you asked. I think the answer is vaccine companies are very conservative, and vaccinologists are very conservative. and so. There are a few things we do differently, which I think really, they should all be doing, right?
One is we test a lot of immunogens in vivo. We've tested, as I said, a thousand plus immunogens in vivo, or, you know, something in that order of magnitude for, for our novel vaccine constructs. We have characterized the response to all of those immunogens, and we use that to sort of decide what we want to advance into, into clinic, right?
A typical vaccine program, as far as I understand from people in big pharma, really doesn't test more than a dozen or a couple of dozens of these in vivo, of these different sort of design candidates to the extent that they design it all, right? Before they move forward into clinics, so they go to clinic pretty quickly in big pharma because they have a lot of money. But then often these designs don't do what you want them to do because you haven't really optimized them to and so they fail.
[01:07:31] Abhi: So, it's not necessarily that they're designing only 10 because they have like such a significantly higher hit rate than like anyone else.
[01:07:37] Soham: Oh, no. And you know, one of the reasons big pharma has pulled back from vaccine investment is every big pharma company, almost all of them have some like crazy failure story where they invested a billion dollars in a vaccine program and it went, it went just super wrong, right? Like Sanofi has this Dengue vaccine program that we were talking about before this, where similar to RSV, when you take their vaccine and you haven't been exposed to dengue before, it actually makes your case of dengue worse, right?
And they invested like a billion dollars into that program, which is effectively money set on fire, right? And had they been smarter about precision immunogen design. I think they wouldn't have made that screw up.
[01:08:15] Abhi: Is, do you think, like prior, like, like pre 2021 precise immunogen design was just like off the table for anyone?
Like, like prior to the release of AlphaFold 2? What are options for people to do precision immunogen design before?
[01:08:28] Soham: Not in the way that we're doing it, but certainly there are ways to sort of rational design using Rosetta and tools like that. And I think there are ways to sort of more comprehensively characterize the outcome of, you know, of, of injecting your immunogen into it's a, whether it's a transgenic mouse model or an organoid model or something closer to humans, I think that was available with a little bit of work.
Right. And I think they could have, I think not just them, but I think lots of people could have done better about advancing ways to characterize those responses comprehensively to figure out whether the response you're getting is actually the response you want.
[01:09:02] Abhi: Yeah.
[01:09:02] Soham: But I think, someone recently, you know, someone who has spent many years in big pharma was telling me, look, the vaccines aren't sexy.
And so preclinically big pharma doesn't invest enough money in like the cool new technologies for, for vaccines. Right. And, you know, the people who are the most ambitious about working on new and interesting stuff often leave vaccines, whether it's within a big pharma company or broadly in the field, wouldn't you rather work in a field like cancer immunotherapy, where there's so much more money and so much more application of the latest methods, right?
Whether it's like single cell transcriptomics, or it's, you know, these machine learning models for protein design or whatever, right? Rather than like a backwater, like vaccines, right? And so I think as a result, you end up with like fairly conservative people doing like a small number of tests, not really comprehensively characterizing the immune response, like a systems immunology perspective either.
And also just often getting it wrong about what they should be designing for in the first place, right? So like we've had this legacy of HSV2 vaccines trying to design for neutralization. Where it turns out, and there's a lot of good data that shows this, neutralization is not sufficient, in order to, to be able to clear HSV 2, or to prevent, HSV 2 infection.
You need effector function. which is something that people have known now for a little while.
[01:10:22] Abhi: Sorry, could you, like, like, walk me through the, like, the rationale for that?
[01:10:25] Soham: For what?
[01:10:25] Abhi: For, like, why you need effector function.
[01:10:28] Soham: Empirically.
[01:10:29] Abhi: Okay. Just like, that's just like,
[01:10:30] Soham: I don't know why,
But like empirically you need a factor function, like you, you need to be able to have these, like, you know, some kind of ADCC (Antibody-dependent cellular cytotoxicity) or, you know, ADCP (Antibody-Dependent Cellular Phagocytosis) or something like this, you know, to be able to, to, to clear, HSV2 or to prevent HSV2 infection.
And what happens is, what happened is, I think, people assumed that, that neutralization would be okay, would be sufficient. And so there are a bunch of in clinic HSV2 vaccine programs that were conditioned on like, oh, we got a good neutralization response, and it just turned out not to be good enough, right?
And with tuberculosis, we have a TB vaccine program that we want to start, right? People have been focused on T cell responses for a very long time, and there are good theoretical reasons, like it's an intracellular pathogen and so like maybe antibodies won't do anything, right? But we actually do know that there are antibodies that, that have, you know, substantial effect on, on the bacteria, right?
We know that there are people who have profiled T cell responses from people who are able to clear TB and found that actually the T cell response that matters most, are like helper T cells that essentially help the elicitation of antibodies, right? But nobody really seems to have a TB vaccinology program based on antibody elicitation, funnily enough, right?
And when we propose this to people, like, old hats in the room say like, oh, we know it's T cell response. Well, if you know it's T cell response, why 30 years? Yeah, why don't they work? Exactly.
[01:11:58] Abhi: You've mentioned before that, like, immunologists are like, Like not very trusting of like empirical data, how like, like, like, do you, do you think that like the distrust is like a little bit rational in their eyes?
Have they been burnt before a lot on like,
[01:12:12] Soham: So I don't think I said that. Let me actually rephrase what you said. What I think is that they're not sufficiently empirical in that I think they're not willing to try enough stuff and then just sort of like systematically figure out what works and what doesn't.
So like, I think what's really worked for me as a non biologist, like as a computer scientist is like, I don't a priori have any pet theories about what works and what doesn't, right? Like, I'm very driven by like, okay, what are the possibilities of what could work here, right? And for that, you do need to know some immunology and, you know, I asked the right people, right?
And then I'm just like, okay, let's make designs that are going to optimize all these different things separately. And let's see what works best. So if you're an immunologist and you know, effector function is a thing. Okay. Right? Or if you're an immunologist or a vaccinologist working on TB and you know that antibodies are a thing, why wouldn't you just have another, a design that focuses on antibody elicitation also, right?
Why wouldn't you want to like systematically just create a matrix of all the things that could matter, and then make designs that could optimize in those directions, and then see what works and double down on that, right? I think that's sort of the recipe for what we've done at PopVax that works super well, right?
And that, you know, strangely enough, I think a lot of people who are immunologists or vaccinologists aren't doing. I think they come in with some theory about how this vaccine is going to work. and then they make a very expensive, multi year, maybe even in some cases multi decade bet on that theory being correct, without having any backup options, or without even really comprehensively comparing it to other alternative hypotheses that might be the case.
[01:13:41] Abhi: Instinctively the reason, the reason why they would do that is because they have so few shots on goal that they need to like have some very strong prior.
[01:13:49] Soham: But preclinically, they have lots of shots on goal.
[01:13:51] Abhi: That's fair.
Yeah.
[01:13:52] Soham: And even phase one, frankly, they have lots of shots on goal. So like , in phase one, right?
I think you would still be able to distinguish between a strategy that might work much better in phase one, because you could take that, you know, you could, you could see that, it's eliciting effector function. Maybe that's good. Right? Like maybe that, that's going to do something. Whereas it's neutralizing, but like, you know, it's maybe weakly neutralizing or sort of not giving us, complete clearance of the pathogen, if you passively administer those antibodies into the animal model, right?
And so there are all sorts of things you can do if you have some early stage data, which isn't terribly expensive. Whereas instead, these folks are jumping straight into phase two, phase three of hundreds and hundreds of millions of dollars. And then that money is all set on fire.
[01:14:38] Abhi: Is that just because like, overconfidence, like what's causing that like clearly like mistaken step?
[01:14:47] Soham: I don't know. I don't, I'm not these folks and they're very smart people who know more than me. And so it's possible they have very good reasons for what they're doing. But in practice, I think what we see is, you know, there's like a, there's a lot of resistance to trying out things quickly, right?
And in the field, and I don't see why, you know, in addition to whatever theories they have, they wouldn't be benefited by just trying out more things quickly.
[01:15:11] Abhi: Like orthogonal theories, just like to have backups.
[01:15:14] Soham: Yeah, yeah, and then maybe you can combine that, right? Like, you can combine the immunogen that gives you really good neutralization, good effector function, and then you put that in.
Whatever the mechanism is by which it eventually works, it's probably more likely to work.
[01:15:27] Abhi: Instinctively. I imagine that like. I kind of, like, assumed, like, the focus on broad protection, like, goes without saying. Like, every vaccine company is interested in broad protection versus just one variant.
[01:15:36] Soham: But they're not.
[01:15:37] Abhi: Yeah, like, that's interesting. Why, like, like, you know that the virus is going to mutate.
Right. Like, why wouldn't you try to, like, design for, like, highly conserved epitopes?
[01:15:47] Soham: Well, so, designing for highly conserved epitopes and actually achieving broad protection are very much not the same thing.
[01:15:52] Abhi: Oh,
okay. Right. Well, why is that?
[01:15:54] Soham: It turns out just a naive strategy of designing for conserved epitopes like doesn't work, right?
Okay. And so because this has been hard, I think traditional vaccine companies have shied away from it where, you know, what you can do reliably is you can do high multivalency, for example, or you can show as in the case of pneumococcal vaccines, you know, you can show 20 serotypes, right? And, and thus get a response against all those and at least reliably protect against those, right?
But in the case of influenza, we don't even do that. So influenza vaccine platforms, typically use egg based production. That's what most influenza vaccines are made in. And, you know, the problem there basically is, it's difficult enough to even optimize two or three or four strains in the eggs, which is up to four valence is what seasonal influenza vaccines have been, and, it's, the strains that are recommended are often not the strains that work well in eggs, so you have to find some close by strain that, that produces well in the egg.
Scaling that up to high multivalency is very hard. So even broadly protective influenza vaccines haven't really been made, right, that are these highly multivalent vaccines like PCV. But yeah, actually designing vaccines for broad protection using conserved epitopes requires the kind of precision design strategy that we've been talking about that we do at PopVax because naive approaches to it just don't work.
[01:17:15] Abhi: My naive assumption of like, you take your virus, you align it to the nearby virus families, find the conserved epitopes, turn that into your immunogen. Why, why, why doesn't that work? Why is like the not just focusing on the most conserved epitopes, not a good strategy for design?
[01:17:29] Soham: It's just not sufficient, right? So if you look at beta coronaviruses, so like SARS, MERS, you know, these, these pathogens, right? You take the conserved epitopes, there are a few conserved epitopes, and you just make a vaccine. That has those epitopes and I know because we've done it and you inject it, you get something that like doesn't neutralize, and, is no good for protection.
[01:17:50] Abhi: So then what actually causes broad protection? Like, what, like, what, yeah, like, what causes it?
[01:17:55] Soham: So it's not sufficient to just look at conserved epitopes. What you have to do is you have to look at conserved epitopes and then find a way to redesign the, or display that epitope, you know, design the immunogen, right, in a way that actually elicits that neutralizing or effective function or a response that you want.
Natively just displaying the conserved epitopes is not good enough, right? And because nobody has developed the kind of pipeline that we're developing, or that maybe a few others are working on, up till now, and in some sense the technology hasn't really been available up till now, to do this kind of feedback loop, where you have, you know, a whole bunch of computational designs, and you figure out what they elicit.
And then you use that to fine tune your model, right? People have been trying to do this manually, where they, you know, Rosetta, to like manually design the display of the epitope. And that just doesn't work. You're not able to elicit the antibodies that you want, right? And so in theory, oh look, there are these conserved regions, let's elicit something.
In T cell responses it kind of works, so you can get a T cell response against these conserved epitopes. But typically a T cell response is not sufficient to actually give you a protective response against a pathogen. In fact, maybe one exception, none of the infectious disease vaccines that we know work, right, that are licensed operate primarily on the basis of a T cell response. Eventually, it's the antibodies, which, you know, in some sense are early enough to be able to clear the pathogen. A T cell response only like a, say, a CD8 T cell response, definitionally only work once your cell has been infected and then it can clear the cell, which in many ways is too late.
It's the antibodies that can prevent the pathogen from actually entering the cell in the first place.
[01:19:26] Abhi: When, I imagine like the average immunologist does think a lot about conserved epitopes and like comes at it from a very structural biology perspective. Does PopVax ever consider like structural biology, like when you guys are redesigning stuff, or is it very like hands off, black box approach?
[01:19:41] Soham: So like vaccinologists do think about epitopes. Yeah, yeah, yeah. but no, I think it's an important distinction because there's lots of immunologists.
Not that many vaccinologists, right? People actually practically working on the problem of better vaccines, fewer than you would think. Lots of, like, people working on, like, basic science immunology, systems immunology, increasingly. People actually working on practical, like, nuts and bolts vaccine design, not so many, right?
Especially ones with innovative strategies, right? The kind of next, sorry, can you repeat what was the second half of your question?
[01:20:10] Abhi: Like, are the, PopVax is coming at it a very like, like, like black box.
[01:20:15] Soham: Yeah. So we use a lot of structural biology.
[01:20:17] Abhi: Really?
[01:20:17] Soham: So the guy who runs our immunogen design team, co-runs it with me , as a structural biologist by training, not originally machine learning person.
And so, you know, yeah, as I said, our initial approaches were very kind of Rosetta -y structural biology approaches to designing this display mechanism for immunogens, and then redesigning again, using a structural biology approach. How, the, the RBD immunogen in particular for SARS CoV 2, to try and sort of,
to, to sort of better display certain epitopes on it to, to kind of close off certain other epitopes and try and get some of these broader antibody responses we want. And that was all very structural biology influenced. We do a lot of molecular dynamics simulations and stuff, as I told you. The black box approaches we've been able to do more recently, A, because the machine learning models for protein design have gotten better and B, because we've had the money to be able to actually scale up data collection to be able to, to build this feedback loop, right?
But when we started, it was a lot of kind of more manual structural biology approaches.
[01:21:12] Hiring in India
---
[01:21:12] Abhi: And kind of like on this topic of like, creating this whole pipeline sounds incredibly challenging, like meeting GMP standards, building something in India. And as I mentioned earlier, India is very much not a place where a lot of biotech research companies come from.
And as a result, I imagine hiring people is incredibly challenging. But at the same time, the IITs of India almost certainly produce a number of really high quality engineers and scientists. How have you, how have you, like PopVax in general, tailored your interviews to discover these like diamonds in the rough?
And is there anything counterintuitive you've learned about identifying good talent that can do good biology research?
[01:21:48] Soham: So I think there's a lot of good talent in India. Let me say this straight out, right? Just in case anyone is confused about it, there is a lot of good biology research talent in India.
And if you are watching this, you, whoever you are, should fund those people. But, I think what, what we've learned is there is a lot of talent. There's a lot of noise. So there are a lot of people who have impressive looking CVs, whose actual research work is less impressive than they describe it to be, or in the worst case, they haven't really participated in or fully understood the research work as deeply as they're portraying, because there's a lot of top down hierarchical management of PhD students and of, you know, any researcher in India, I think more so than there is in the US or in other places.
So you will encounter researchers who have done cool looking work who like really don't understand the work, right? Because it's the idea of their advisor, their advisor told them what to do, et cetera, et cetera, right? What we've also found is people have impressive looking CVs who can't do wet lab work, right?
And so what we ended up doing to try and screen is we, we have a multi part interview process where we initially start with a casual kind of conversation where we have them, you know, they usually, when they're on the job market, folks in India and in the biosciences, they have some kind of presentation, which they show to people.
We tend to disrupt that a little bit, which is to say we ask a lot of specific questions, and then we sort of, you know, we don't let them proceed in their, their kind of memorized flow of what they intend to do, but rather dig deep into one or two specific things and see how deep they can really get in explaining what and why they did it.
I think a lot of people then sort of are not able really to explain. And so we, we kind of eliminate them from our, from our process. But even, you know, even from that initial screen, I think, we were able to eliminate 80 percent of people that we talked to, right? So we can, we have a large number of initial candidates that we interview for any position and maybe even hundreds, right?
But we can cut those down pretty quickly with like a 15 or 20 minute conversation, right? And we have a large enough team now that we can kind of split the load across people interviewing, right? Then after that, we typically do another, kind of deep dive into some specific piece of work that they've done.
And then we have them come into our lab, and actually do wet lab work. So, especially people who are on our wet lab teams, we, we have all of them, no matter how senior, we make them come and do some assay, right? That we give them the protocol for, and we kind of walk them through the reagents. For people who are in analytical, who do HPLC work, we tell them, look, here's the molecule you have to identify, tell us what reagents you want and we'll procure them for you so you can run this on our machine, right?
[01:24:28] Abhi: I've had friends in the U.S. In wet labs wish they could do something like that, but they always say like it's too expensive to like scale up across many different people. Is it just like the cost is so low in India that it's possible to do stuff like that?
[01:24:40] Soham: Yeah, like the cost of, you know, the cost of, of, of getting somebody in to do this is it's not that high, and people seem willing to do it. You know, we pay for their travel and so on and so forth. But we don't compensate them for their time, which is something we tell them up front, and, you know, they seem willing to do it.
And we, you know, the reagents I think are cheaper than the cost of hiring someone wrong.
[01:25:03] Abhi: Yeah, that's fair.
[01:25:04] Soham: So we've already eliminated most people in the pipeline by the time, by the time we get them to this point. and we find that people are actually excited by the activity, too. Like, you know, many of them have not, they're in some kind of interview process where they're talking to a lot of people, but they not have the opportunity to actually get into the lab and show their skills off.
So the people we want at PopVax are people who are excited to be in the wet lab, right? Who, you know, they have ideas and they can, you know, they can come up with, new designs or they can come up with new approaches to our assays and so on and so forth, but they also want to go and execute those things.
They don't just want to talk about them. And we find that those people are jumping, you know, at the opportunity to do interesting work in a well equipped wet lab and to talk to people about the work they've done. When people come in and they're reluctant to jump into the wet lab, we typically, even if they're senior, even if they have like impressive CVs, we typically take that as a negative signal.
We say, we don't want to hire this person. Right?
[01:25:56] Abhi: Like you've mentioned in the past, like there's like this, the talent in India is like largely wasting away at companies that are not doing R&Dwork. Yes. Is there, are there, like, like particular institutions that like if you see on someone's resume, you're like, oh, they're probably like bored out of their minds there and are very talented.
They should come work at PopVax.
[01:26:14] Soham: Yeah, like if there are people who are at IISc or NCBS or CCMB which are all like, you know, central government, you know major institutions that do basic and translational science work and then we see that they're at a contract research organization and there are a bunch of those in Hyderabad and in Bangalore, where they're just doing the same assay over and over and over and over and over again, or they are at a contract research organization for synthesis where they're just doing chemical synthesis, the client tells them what to do, they don't have any input into the design process. And we see somebody from one of these top institutions, then yes, I think that's a good signal to us that maybe we can pry them away. If we offer them interesting work. And I think that's another advantage for us in India. Hiring is hard, right?
Hiring is hard everywhere. I think canonically it's a hard problem, right? I don't know how it is for you folks at Dyno or like, you know, your colleagues, but, yeah. I think because we are one of the few people in India doing innovative R&Din the biosciences, that's also translational enough that it's really close to being applied.
Like we're in clinic pretty soon from, you know, not, not immediately, but like we'll be in clinic next quarter. Right. We, we have this pipeline of six different new vaccines. We want to take to clinic in the next two years. And so that means if you come in and you do something that has a chance of affecting something that'll be injected into somebody not too long from now.
Right. What we find is as a result, we can motivate some of the best people in the country, to come work with us. Whereas in other places in the world, they would have many options to choose from. And so I think in that way, we can agglomerate a lot of the best talent, right. And become a beacon for the best talent to come and collect.
And not just within India, but talent from outside of India that are Indian origin, but want to return to India, whether for family reasons or, you know.
[01:27:50] Abhi: I was, I was going to ask that as like PopVax have their own version of that, like Chinese policy to bring minds back home, where you guys like do reach out, to a really promising scientist like abroad and like try to get them to come back.
[01:28:00] Soham: Yes. So I spent a lot of time on LinkedIn going like, hey man, like, would you ever consider coming back to India? And you know, the, the two folks you met today from PopVax, my colleagues, Maunish and Darshit are both people, who grew up in India.
Went to undergrad in India, did grad school, did their PhDs outside of India here in the U.S. and Canada. and then, you know, I convinced them, and, I think to some extent they convinced themselves. But also, I was able to convince them and our team and our work was able to convince them to come back to India and work with us at PopVax.
And we have, you know, but, a dozen more people who fit that description who are people who we have convinced to come back to India from other places to work with us, mostly people who wanted to come back to India already had a bias towards that, but that we were able to show, look, we're doing good enough work that you wouldn't waste away if you came and worked here, you would actually be doing very exciting things. And so that has been a big advantage for us, because again, as I said, not too many people in India offer that opportunity. So if you're somebody with a bias to coming back to India, and you want to work on bio, you should work at PopVax, and you should email me at soham@popvax.com.
But, but also I think, you know, they, we are kind of an obvious option, right? As more people hear about us, and as I do more things like this, I think more and more people will reach out to us as they already continue to. So when I was in Boston last time in December and I'll be back in Boston tomorrow.
I had a meet up with about 15 people who you know, on LinkedIn, had reached out and said, I'm interested in coming and potentially working at PopVax. And these are people working at extremely well funded biotech startups in Boston, at Big Pharma, Merck, you know, like Sanofi, et cetera, et cetera. and so I think that, that is a very interesting opportunity that we have that other people can have if they come and start those companies, but they haven't yet.
Right. And so that gives us the ability to recruit maybe a more, substantial density of talent than if we were in an ecosystem, like in Cambridge, where there's so many different competing companies, right?
[01:29:53] Abhi: Like, at least in China now, it feels like over the last few years, there's been a vibe shift of like smart students wanting to stay in China and just like work at like, like Baidu, Deepseek, whatever, interesting companies are in China.
Do you think a similar like that, similar thing like that is happening in India currently, or we're still like a few years away?
[01:30:08] Soham: think this in a limited way. in software, you know, in Bangalore, to some extent, in bio, it's still a lot of people wanting to go abroad because they're just better resourced, better resourced labs, better resourced companies, and they get to do more interesting work.
But I think people would love to come back to India, if given the right opportunity. I think lots of people feel that they're culturally out of place in other countries. Increasingly, they feel there's anti immigrant sentiment. In Canada, I know people who feel that there's rising anti Indian sentiment in particular in the last few years.
So I think lots of people would love to come back, given the opportunity, but they don't feel the right opportunities exist, especially in bio. So I think that's something that in software has changed a little bit. I think in bio too, if we can do a good job of bringing together a coalition of companies who are doing interesting work like this, I think it will change.
[01:30:54] Abhi: I'm curious, outside of like PopVax, do you see any other like really like great R&D effort going on within bio or outside of bio? In India.
[01:31:03] Soham: There are a couple of very interesting antimicrobial efforts like in, in antibiotics. There's a company called Bugworks run by a guy called Anand Anandkumar in Bangalore, which has raised a bunch of funding is doing interesting work in novel antibiotics. There was recently an antibiotic approved in the US. A novel antibiotic which was actually developed in India by a company called Orchid Pharma. Which is like a Chennai based company, boostrapped, that has been doing, and now they're bought, but they're doing really interesting work for many years on new antibiotic development. A company called Wockhardt, which is, an Indian generics player, also has developed a new antibiotic.
So there's some interesting work in that field. In novel biologics, less so, there is, a company called Immunoact, and then another company called Immuneal, both of which are doing CAR-T. And, and largely they've been focused on the, like, cost angles to make it cheaper for folks in India and other rest of world geographies, as they're called, ROW.
But ImmunoAct actually has an interesting technology where it's sort of a more immunized, sorry, more humanized, kind of de immunized, cell that they're using than, than existing CAR T. And so it has a cleaner immune profile, they claim. And I think some of the data shows than existing approved, CAR T therapies.
And so I think that's an interesting, that's actually a novel product. I think it's maybe licensed off NIH, but I think it's a novel product that they've developed and they've got approved in India. But I don't know that they have the intention of bringing it to the U.S. Or bring it to, to, to richer countries, right?
So, I think there's like some beginnings of interesting stuff happening. But I also wish these companies were more ambitious about saying, okay, my product is going to be best in the world. And so therefore I'm going to get it approved, not just in India, but in countries where there's a lot more money to be made, because that can fuel that money will fuel your R&D engine in the future, right?
Cause that's, you know, if you want to make money off biologics, you need to be approved in the U S right?
[01:32:51] Abhi: Yeah. It's like, and that's actually kind of related to a question I was just about to ask. PopVax is starting your phase one trials that are going to be ran by the FDA using
[01:32:59] Soham: No no. NIH.
[01:33:02] Abhi: And that does lead to a question like why focus on appealing like purely to like US based markets or like US based like federal regulatory agencies. Is this, is that just like more valuable for you guys?
Does India have issues with approval of novel biologics or something else entirely?
[01:33:19] Soham: So I think, a few different things. One is obviously it gives us a lot of credibility to get phase one data in the U S. I think people still ask questions about the credibility of clinical trial data in India, which is unfortunate.
And there are some reasons for it, but we want to be able to say, look, you know, this data is unimpeachable. And if NIH generates the data, it's pretty unimpeachable, right? So that was a very good opportunity for us. And you can't really beat free, right. You know? But I think the other reason is, yes. the Indian regulator is undergoing, I think, a process of evolution.
I think at the moment they are harder to get approval, for new phase one trials in India than it is in the U.S. And often they have more, they object in ways that, you know, they ask for animal data that takes a long time to generate, that isn't necessarily consistent with what current sort of modern standards would say.
So a good example of this is, we've not done NHP studies, you know, primate studies for our vaccine. That's because FDA told us we didn't have to. But to do the phase one in India, it's almost certain that we would have to do NHP studies. Even though it is extremely difficult now and expensive to do NHP studies in India because of their sort of other animal regulation issues that have made that difficult.
And so in fact, it's actually easier for us to get a slot in an NHP lab in the U.S. In terms of just time than it is to get one in India.
[01:34:32] Abhi: Is this unique to like kind of like the novel modality you guys are approaching or like even like small molecule drugs?
[01:34:37] Soham: I think it's worse for the novel modalities. It's like biologics.
[01:34:40] Abhi: Really?
[01:34:40] Soham: It's much worse. And so there's a lot of education of the regulator that has to happen because they haven't seen this movie before. Whereas the FDA has seen this movie before. They've seen the sequel that you haven't seen yet because they've, you know, they've previewed all this data. And so they can actually, I think, be very astute.
And that's the other reason the FDA, I think, is very valuable is we did a pre IND with FDA, where we basically wrote them a bunch of questions and they give us a bunch of really detailed answers. And in those answers, implicitly, I think was the accreted knowledge of seeing a lot of mRNA trials and really understanding where we should be looking out for safety risk, which is what you care about in phase one.
Whereas in India, there have been, there's been one mRNA vaccine that has gone through trials, right? Which is quite different from ours in many ways. And, the regulator just doesn't have that knowledge yet. It will, they will. I mean, they're smart people, right? So I think what we hope for, and I think this is one of my other policy prescriptions for India is, like China has done, we were talking about this briefly earlier, China has made it much easier for people to do Phase I's.
Maybe made it too easy for drugs to be approved, some people would say, but I think Phase I's should be really easy to get approved. Australia has this really cool regulatory framework where they don't even, you don't have to apply to the regulator, you can like, have these sort of, decentralized, ethics committees basically make the call for a phase one.
I think some system like that in India would be a huge boost to biotech research and would really marry our, similar to China, like marry our ability to do really cheap trials, with the ability to do really fast trials, especially the early stage, and develop data for assets that we could then, you know, whether it's actually get them approved in the U. S. or market them to, you know, to, to U.S. big pharma companies for collaboration and so on and so forth, as Chinese companies are doing. If the Indian government doesn't want to do that across India, they could do that in a in an SEZ, or they could do that in like some kind of special zone where every like, you know, historically, the problem in India has been, and I empathize with this, has been, you don't want people from other countries coming in and using our people as cheap guinea pigs.
And I feel this, I don't think we should be doing that, right? I don't think that's what we're trying to do, right? We're an Indian company trying to develop new drugs that will help India, you know, develop new vaccines that will be valuable for infectious diseases in India, right? But, I understand the concern.
And so I think therefore having some kind of special zone maybe where when you go in, you basically sign a, a series of documents that explain that, you know, you have maybe more education than average. You have sort of more ability to understand what's going on than the average person in India. And so therefore you're, you know, kind of a de risked population in which to, to, to do this, right?
[01:37:09] Abhi: Do these, like, do these SEZ's exist? Within India for any other industry?
[01:37:12] Soham: There have been talk about doing, for example, medical SEZs for medical tourism, where the laws are somewhat different, would be somewhat different in terms of qualifications and so on.
I don't think it's happened yet, but I think that the framework certainly exists to do something like this, where there are SEZs for, there are SEZs that have very different regulations, like GIFT city in India for, for financial, you know, and, and, and sort of, you know, foreign exchange purposes. So there's no reason that you couldn't do this as well, right?
And so I think that if, if you did that, you could build in India, a biotech research ecosystem end to end where it's like, we can do the early stage work, you know, the discovery, we can do the phase ones, right. And, you know, potentially even later stage trials where you can generate. all this really good data, and the Indian population is quite diverse, so in a quite diverse population of whether these, you know, novel drugs that you're designing work, right?
And that becomes a very then compelling case to be able to take those cost structure advantages and use them to just get faster and faster at developing the best biologics, right? And that's what we're doing at PopVax to some extent. It's 10 times cheaper for us to do a lot of the wet lab work. It's 10 times cheaper for us to do animal work.
And so that means for the same dollar, I can do either 10 programs or I can potentially go 10 times as fast on the same program, right? And eventually, we will just leapfrog what a company like Moderna is able to do because, you know, they can't keep losing the billions of dollars that they're losing forever.
If I'm just way more efficient, I think I'm a better bet. I'm a better investment, in terms of being able to generate good outcomes for for new vaccines and from a philanthropic perspective as well, right? You know, we work with our philanthropic partners.
[01:38:48] Abhi: Like dollars are better well spent here.
[01:38:49] Soham: Exactly. Yeah, dollars per life saved. I think, you know, if you even if you assume we have the same hit rate as Madonna, why would you give them 10 X the number of dollars to develop the same drug? And in fact, I think we will have a better hit rate, right? So, like, you know, I could be wrong about that, but I don't think I'm wrong about the cost structure.
[01:39:05] Abhi: Mm hmm.
Yeah, I mean, that feels like empirically true as the last three and a half years I've demonstrated.
[01:39:09] Soham: And I think in particular, right, why Big Pharma is pulling back from vaccine investment. We talked about this briefly. One of the reasons is it's a billion dollar program and it won't generate as much money as a cancer immunotherapy or a GLP 1 because it's only dosed once and it, you know, it costs like a hundred dollars or something, right?
Which is why, by the way, anti vax people who say like, you know, vaccines, oh, pharma companies just trying to make money off vaccines, pharma companies don't want to make vaccines. they, they want to not invest in vaccines. But I think with our cost structure, if I can make a program cost one fourth or one fifth as much, we'll use, I still end up spending a lot of money on late stage clinical trials in the U.S. or whatever. But maybe you do that after you've already got approval in another country for a completely novel vaccine. And so now that's de risked to get that next round of capital to do that. I think I can do a program at one fourth the cost, right? As a big pharma company can do that. And if I'm also better at getting a more efficacious product through the door, if my hit rate is higher, right?
I think that combination makes vaccines not just investable, but a highly, highly profitable endeavor. Because the best vaccines still make, you know, two plus, in some cases, eight billion dollars a year, right? And so if we can be a company that makes the next generation of the best vaccines, then and just because we have a repeatable engine to be able to do this cheaper and faster, you know, we can be a pharma company that's the size of any global big pharma company, but then because we have the same amount of money as they do, and we have access to this cheaper R&D base, I can suddenly do 10 times the amount of R&D.
And if this keeps compounding, you know, eventually it'll be a, I think a huge win condition for the world and for us in India and biotech R&D.
[01:40:44] How fundraising for an Indian vaccine design startup is coming along
---
[01:40:44] Abhi: On the subject of like funding at all, I know that, when, PopVax, first started, I think you guys primarily survived off of the Gates Foundation money.
I think historically, as far as I can tell, PopVax hasn't ever received money from an Indian organization.
[01:41:01] Soham: No.
[01:41:02] Abhi: Is that, yeah, like, why has there been, like, such hesitation, like, over three, three and a half years?
[01:41:07] Soham: We haven't looked that hard recently, so I can't say recently what the, the outcome would have been.
The government funding available to us was too small.
[01:41:15] Abhi: Gotcha.
[01:41:16] Soham: So it was, like, 50,000, sorry, 50 lakh rupees, which would be the equivalent of somewhere between, like, somewhere in the 60,000 dollar range. It was sort of the prototypical grant that you get at the early stage from BIRAC, which is this organization I'm talking about.
It's not nothing for, you know, doing work in India, and it's good that they give that out. But yeah, it wouldn't have moved the needle for us. Beyond that, it was difficult to find funding from the government that didn't require that we were at a TRL, like a Technology Readiness Level that was much higher than we were actually at.
Right. So like, which is basically like, you need to be in phase one or you need to be sort of like already very close to phase one, which we weren't three years ago. Right. Private funding in India, we did try and we were very disappointed. Like we heard a lot of people tell us either it was impossible. or we had people tell us that, you know, the government should be funding this.
I heard a lot from like billionaires. I was like, you, you live here, like, you know, you know that the government doesn't fund this. Or at least not as much as governments in other countries do. And you know, perhaps there's an opportunity for that to change. And maybe there are people working on this.
You know, I know there are lots of smart people who have tried to push policy in the direction of funding more R&D, but it hasn't happened yet, right? And then Indian venture capitalists, we didn't, again, pursue very seriously, but the early conversations we had just to understand, whether this would be worth pursuing, were that because they did not have biotech folks, they would not be able to sort of, you know, assess the technology in a way that would allow them to lead a big round.
I think if we had been smaller and we needed less money when we went out to raise, it would have been possible to get from some small funds that now think about biotech, like small amounts of money, like a million, two million. But you know what we need is more than that. So when we when we started raising recently, it's a Series A that's much larger than that.
And so that, I don't think, is yet something that these funds are like the funds that have the money in India are able to do. So, yeah, we just. You know, we tried, we, we didn't get money from those people. We got money from other people. So most of our money, in the very beginning was Gates money, for this, for our platform and for our next generation COVID vaccine, where the intention was basically, make it such that it's able to be broader and it's able to protect against, multiple different variants that might emerge rather than sort of becoming useless after a few mutations, right?
And also to try and make the vaccine more thermostable so that it could be distributed in developing countries, right? Then after that, the next big chunk of money was from Vitalik Buterin, who's the Ethereum cryptocurrency co founder, who's very interested in biosecurity, and in sort of open source, public health work, where like, how can we make these things really accessible in a way that people can not only buy them, but also sort of develop further on top of this.
And so our COVID vaccine, which is funded largely by his group, Balvi, is actually open source. So we're going to put out all the information about how to make it. We're going to allow people to, to make changes to it and, and sort of make new versions of it without enforcing our intellectual property on that specific vaccine.
Though we'll be able to use our IP for other things, other vaccines that we're producing, right? And so that was also a weird funding mechanism that I said, you know,
[01:44:31] Abhi: I mean, alongside like the, like how like the financial barriers in india are just so much lower for vaccine development compared to everywhere else. You have a few articles published on your Substack about some of the results you guys have had over the past year or so and they feel like clearly extraordinary when you look at them.
[01:44:46] Soham: Thank you.
[01:44:47] Abhi: You have an influenza vaccine that has better IgG and HAI titers compared to your competitors.
[01:44:51] Soham: 250x better IgG titer is the headline result for H1.
[01:44:55] Abhi: Which is insane and those antibodies still remain effective against mutated influenza strains?
[01:44:59] Soham: Yeah, so this is pre clinically, so this is all in mice to be clear, but like, yeah, so we, we found our influenza result is that, our version 3 influenza seasonal vaccine construct, it's not actually version 3, it's many more versions than that, but sort of, you know, major version 3, is 250x better in terms of H1N1 IgG titer elicitation and also way better for, for H3N2, those two of the key seasonal strains in the vaccine, like more than 100x better, but also is able to, yes, as you said, elicit robust IgG titer against H5, H5N1, even though it does not contain, the H5 antigen.
[01:45:29] Abhi: Yeah.
And so like, like looking at this on face value, you've made something clearly that people want, and it seems clearly better in every capacity compared to your competitors. Yet, you've told me that fundraising is a continuous challenge. Is this just because like vaccines are like a, like a hard deal for anyone trying to work in this space, or do you think there's like some level of like, like VC education or like government education that needs to be done?
[01:45:50] Soham: So two things. One I'll say is since we spoke last, the last week of, of investor meetings have been, have, have, I think we've turned a corner, I think, I think in part because of this data, right? I think we will be able to, you know, I think it's now materially easier for us to raise money. And it's because, you know, as it should be, because we have really good data.
And we also won this 2 million BARDA award, as part of their Patch Forward Prize, in part based on this influenza vaccine data. And so BARDA, which is the U.S. Biomedical, sort of the biodefense agency in some sense, but in HHS, is, you know, both a potential funder of future influenza programs for us and a potential customer of pandemic influenza vaccines.
And so I think that creates a setup in which people are much more willing to take seriously the idea that, actually, these guys might be in the future, one of the best influenza vaccines, right? But I also think we are at the intersection of three different things that people don't understand. One is, we're an Indian company doing novel biotech R&D.
It doesn't exist.
[01:46:46] Abhi: Yeah, I talked to multiple biotech VCs in preparation for this, and none of them had helpful advice to give because they've never met, like, a biotech founder or biotech, like, interesting biotech company that came from India.
[01:46:57] Soham: How, how skeptical were they that it's possible?
[01:46:59] Abhi: Like, they were all actually like pretty curious.
[01:47:02] Soham: Oh, really?
[01:47:02] Abhi: Yeah, like none of them like wrote it off immediately. They were all like, yeah, like I've never thought about it, but I've never met one.
[01:47:07] Soham: Yeah, but they've never had one, right? So clearly I'm not wrong that like almost no one does this. Yeah, yeah. the second thing I think that, and so I think part of that is like, is there, is there a talent gap where you just can't find the talent in India?
And the second question is like, is the data reliable? Because the China had all these data issues and we have data issues in India and so on. And I think the answer to all those questions, it can be done. You just have to be very careful about how you do it, right? The second issue is, yes, vaccines, people for many years have not invested as much in vaccines. COVID was this big spike in vaccine investment. but it felt, it's like a bit of a sugar rush. Like after that, it's like the, the fall has been precipitous. and right now, Moderna, is being hammered for being an infectious disease, Pfizer is being hammered for being an infectious disease company. And I think that's because people think historically, these vaccine investment cycles are very long and, you know, probability of failure is high is the typical thought process. And obviously we think we're different in that regard, right?
But that's also some education that has to be done and some understanding of what the new cost structure looks like. And the fact that we think we can be much more likely to succeed than existing vaccine design approaches. The, the third thing that is a. you know, as an issue is that the biotech funding market as a whole, as you likely know, has been a bit bad, right?
[01:48:16] Abhi: It does seem to be getting a little bit better recently.
[01:48:18] Soham: A little bit of a new dawn, it does seem like. But you know, for two years, it was quite, it was like really as bad as anyone had ever seen it, right? I think, and public biotech stocks are still not doing so hot, right? In many ways.
So I think the intersection of all those three things made it a little bit difficult for us to fundraise. But I think the main barrier that has now been crossed is, in the very beginning, right? If you met me when I started this company, I think a lot of people believe that the probability of success was not high, right?
Because I didn't know any biology, right? And, you know, I was starting this extremely technical biology company in a part of the world where people didn't know a priori that there was really good bio talent, right? So that was, you know, the initial piece of skepticism. I think now I know enough biology and I have a wonderful enough team around me that people are not as skeptical, that I am the right leader for this and that my team is the right team for this, right?
The other big thing I think was, like, can you show that your vaccines are actually much better than what existing vaccines are, you know, are able to do in mice? And I think that's something where, starting with our COVID data, like, you know, about a year and a half ago, and now with this influenza data, we've been able to show repeatedly, oh, we can do this much better, at least preclinically, than, you know, then companies that are much better funded than us that have spent much more time on this problem than us.
And then I think the U.S. Government coming in, right, so like NIH, as part of Project NextGen, being willing to do our COVID vaccine trial, and then BARDA now giving us this award shows that I think, you know, in some sense, the people that know best about this kind of stuff, the scientists that are, you know, I think the alignment of their motivation is very clearly, like, they want better vaccines, right?
If these people are putting some support behind this, then I think that has sort of woken up, a lot of investors and also partners, like, you know, commercial partners, that have reached out to us, like, big, big pharma companies who are like, oh, this is real now, like, clearly, like very smart people have vetted this and have spent some time thinking about whether these would be valuable sort of new vaccines or new vaccine designs for, for the United States, which is the market which makes the most money and the answer has come out: yes. Right. And so I think that was the last piece and sort of giving us the credibility needed for us to say, like, look, this whole thing may seem super weird to you, right? Like the construction of this company and where the money has come from and like who started it and where it is. It may all seem super strange, but the data can't lie, right, you know, at least preclinically, the data is quite repeatable and we can show it to people.
Right. Whether it translates to humans, we'll have to see, but, you know, we're really doing something that's quite different here. And lots of smart people, you know, in the U S government, who are, you know, motivated to get better vaccines are saying that, hey, PopVax is maybe one path to better vaccines for, for Americans.
Right. And I think that has been the, the, the validation that has, you know, made other people jump in and be like, oh, okay, this might be a real thing. Let's jump in and maybe fund or maybe partner or whatever.
[01:51:08] Abhi: Okay. So JPM was a big mental update.
[01:51:11] Soham: Yeah. Yeah. Well we'll see, I think still vaccine funding is such a small subset, like, okay, if I'm a cancer immunotherapy company and we may do cancer, you know, immunotherapy with many of the same ideas that we're doing for vaccines today.
Right. This idea of an immunology foundation model, or at least immunology models that can model elicitation, is also, you can see, sort of intuitively valuable for cancer immunotherapy, right? We're not doing that as our main thing today, right? If I'm at JPM and I'm a cancer immunotherapy company, then I'm the belle of the ball and everybody loves me, right?
Or if I'm a GLP 1 company or, you know, I'm doing something in metabolism, right? With vaccines, there's a small number of investors and partners. It's not all the big pharma companies. It's not all the investors. But I think the good thing is those people, the people who do vaccines, they really understand vaccines.
You know, there are people who've worked on big vaccine programs, who've worked on vaccine research. And if you're committed to vaccines, then typically these funders have built actually big teams, at least, you know, large enough teams, that really understand vaccines scientifically and understand the market.
And so we can have very, you know, high level, sorry, I don't mean high level, I mean rather detailed, you know, and sort of very granular conversations with them about the data and about our approaches, and they really understand those things, right? And so, I'm happy to work with those people, and we'll see which of those people we end up working with in the long term, right?
But it's certainly not most biotech investors, right? Like, most biotech investors. Like, we went to a party at JPM, which one of, you know, with, with one fund that is interested in biotech that invited us to it. And, you know, my colleague Manish was asking people like, hey, you know, would you be interested in talking to us?
We work on vaccines. Just by the by, right? While getting drinks. And, you know, they would be very interested when we said machine learning. And then as soon as we said for vaccines, they were like, Oh, we don't care. Our investment thesis is that we don't invest in vaccines, yeah. Or we're like, we were burned investing in this vaccine company, so we just won't do it again.
[01:53:10] Abhi: Is it because like, like largely because they don't understand the cost advantage of like the fact that you guys are in India? Like, like the terms are a little bit different.
[01:53:18] Soham: The conversation doesn't even go that far. It's like immediate right now.
[01:53:21] Abhi: Okay.
[01:53:23] Soham: I think if we show we can take programs to like phase two, phase three at that cost structure advantage.
Then I think the game will open up. But right now we need true believers. I think in like vaccines, we want to work with people. We believe in vaccines. We want to work with people who believe in vaccines. And I think one big learning for me is like, I don't want to spend my life right now convincing people who really don't believe in vaccines to believe in vaccines.
And then back us because they won't be good partners for us in the long run. Right. Tomorrow, let the like, you know, the johnny come lately is, you know, when in the future when I need way more money, you know, we'd be happy to welcome them in. But at this early moment, because we've not taken any equity capital, right?
So like, I don't have any investors on my board or anything, right? The people that I want to partner with, I want to partner with people who really care about vaccines. And they don't have to be people who have invested in vaccines before, but people like understand that vaccines are a valuable modality.
Both from a public health perspective and a financial perspective, and that there is a way, and people have made lots of money off vaccines, right? You know, there's a company called Vaxcyte right now, that you may have heard of, that is doing a competitive pneumococcal vaccine to Pfizer's 20 valent vaccine, they have a 31 valent vaccine.
And that, just on the basis of phase 2 data for that vaccine, they're at, like, a 10 billion valuation in the public markets, right? Because there's a clear market for that vaccine. They made something that's clearly better. I think what gets lost in the fog of this, is that vaccinology, because, you know, because it's hard and because people have taken these outdated approaches, lots of vaccine companies have failed, not because of the structure of the market being bad, but because it's a hard problem and their approach didn't work.
[01:54:58] Abhi: Yeah. Do you think they're approaching it perhaps in like a fundamentally wrong way?
[01:55:02] Soham: In a fundamentally wrong way, and these are, you know, they, they took too long in some sense to go validate their hypotheses, right? Whereas we want to be able to validate both preclinically and in clinic lots of our hypotheses very quickly.
[01:55:15] How is PopVax so good at designing vaccines?
---
[01:55:15] Abhi: And I think like, maybe, like, relatedly to how, like, other people are doing it incorrectly, and you guys have clearly, like, created something that's better than a lot of other people.
[01:55:22] Soham: Well, to be clear, we don't know if we're doing it correctly yet, until we see efficacy in humans.
[01:55:25] Abhi: Sure, sure.
Like, mouse, mouse efficacy seems to be quite good.
And on the, like, there's, like, enormously better immune response, like, to, like, 250x, like you just mentioned.
[01:55:33] Soham: It's much higher.
[01:55:34] Abhi: Yeah, yeah, yeah. What, what does PopVax do differently for your vaccine design? Whatever you do, why isn't, why isn't that there's, is it like specific to this, like precise immunogen design?
Is there something else?
[01:55:44] Soham: It's like, like, I think we take an engineering approach to the problem.
[01:55:47] Abhi: Okay.
[01:55:47] Soham: Right. Like I said, we do this, you know, mRNA encoded display on these self assembling particles. We do, this precision immunogen design. We do design of our own lipid nanoparticles. What we find is when we co design all those things together and we can just have a bigger combinatorial space that we can test empirically, which is our secret sauce, quote unquote, not very secret, right?
We can find parts of the design space that maybe haven't been tested before by other people that in combination you get these really good results.
[01:56:15] Abhi: Because other people don't have like, don't have like the knobs to tune on those because they're outsourcing it all?
[01:56:20] Soham: Yes, they don't have the knobs to tune and they don't have the wherewithal to test all this different stuff.
[01:56:24] Abhi: Gotcha.
[01:56:24] Soham: Right? And I have all the knobs, right? All the knobs are in my control. And so, and we find lots of wacky stuff, right? We find that there are certain lipid nanoparticle formulations that work better for certain vaccine designs. Mm hmm. Do I know why? No. Is my team trying to figure out why? Yeah, totally.
But does it matter? Right? It, as long as I can find the right set of knobs to tune that give me something that's better. And then, and critically, I can translate that into humans as well. And we'll have to figure out some translational process, using these organoid models and transgenic mice, potentially, which we're doing now.
And using human data we collect in phase ones, and we want to do a lot of phase ones as well, to be able to make that sort of translational gap narrower between the the models we're using now on actual humans. I don't need to, you know, I don't need to, mechanistically explain everything exactly that's going on, right?
I, because within the design space, there are going to be lots of little tweaks, which we don't know exactly why they're better. Right. But we will be able to see in practice that they are better. I think antibody design is kind of the same. I don't know. It's probably similar for you guys with AAV's as well.
[01:57:30] Abhi: I feel like it may be the case for all like AI biotechs were like they were primarily built by like people who have an engineering mindset as well. And they're, they are happy treating the problem as a black box. Like, yeah.
[01:57:40] Soham: And I would like to, once we have something that's better and I advanced that, at the same time, I want to figure out the mechanistic insight if I can use that to inform the next design.
But, I think we have to be extremely rigorous about being empirical and testing, you know, the, the biggest design space that I think will be useful for us, which is much bigger than other vaccine companies have done. But I don't think we have to be perfect about mechanism.
[01:58:04] Abhi: Yeah.
[01:58:04] Soham: I think lots of drugs, people like don't really know the mechanism like they say they do.
But I think a lot of like a lot of drugs like the mechanism is not fully understood right or until later. Yeah.
[01:58:14] Abhi: For, for vaccine. Is that true for vaccines as well? Or is vaccine. Like typically, vaccinologists really want the mechanism of action elucidated before.
[01:58:20] Soham: No, so like there are lots of vaccines like, HPV.
It's like
[01:58:24] Abhi: that mechanism is not fully like,
[01:58:26] Soham: Yeah. So like there's no, what's called a correlative of protection where like, if you get this number up, then you get protection. We don't know what that is for HPV vaccines, and the reason is very interesting. It's because HPV vaccines are too good, so for the strains in which HPV vaccines do work, and there's some space to be explored in making them broader, right?
But HPV vaccines, in the strains that they are designed for, right, in the genotypes rather, they're so effective, even actually in one dose. Even though they're originally a three dose regimen, that you don't get a gradient that you need to be able to say like, oh, you know, it's, it's only partially effective here.
It's more effective here. It's more effective here. And so it's actually, it's this variable that gives you the nice linearity, you know, between that variable and effectiveness.
[01:59:10] Abhi: It's just like a step change.
[01:59:11] Soham: It's just like, it works. And so because of that, we don't really know, whereas in the case of flu, Because the vaccines are often shitty, we do actually know that hemagglutination inhabitation titer and pseudovirus neutralization titer both actually independently correlate with protection.
[01:59:27] Abhi: Interesting.
[01:59:28] Soham: Right? Isn't that cool? So like, we don't really know. Is it effector function? Is it neutral? People think it's neutralization. It could be lots of different things. Even in influenza vaccines, effector function is not usually used as a correlate of protection. Good evidence that it actually matters quite a bit for final disease phenotype and severity, right?
[01:59:45] Pet theories on immune mechanisms
---
[01:59:45] Abhi: You mentioned, like, I think we at some point discussed about how, like, immunologists don't seem to, like, sufficiently update strongly enough on new, on new outcoming data.
[01:59:54] Soham: They do not, yes.
[01:59:55] Abhi: Are there, like, specific, specific theories about immune, like immune mechanisms for some disease that you like strongly buy into, like, like perhaps PopVax is not working on.
[02:00:08] Soham: Sure.
[02:00:08] Abhi: Like you would want like people to be aware that like this particular.
[02:00:11] Soham: So like TB, right?
Tuberculosis, which we do intend to work on.
[02:00:14] Abhi: Okay, yeah.
[02:00:15] Soham: We think antibodies play a big role. If you look at Babak Javid's work at UCSF, he's shown that there are functional antibodies against TB, that, that you can use to potentially clear the pathogen, right? Okay. Nobody's working on vaccines to elicit those antibodies for TB and it drives me insane.
[02:00:31] Abhi: What do they typically, like what do they typically work on?
[02:00:33] Soham: T cells.
[02:00:33] Abhi: T cells, okay.
[02:00:35] Soham: Yeah, 30 years of T cell vaccines for, for TB. And it's like, it's
[02:00:39] Abhi: Never worked, so.
[02:00:40] Soham: Yeah, like there's a study, the Gates Foundation and a bunch of others spending like almost 500, like 300, 500 million dollars, some crazy amount on a multi country study for this one, this, you know, relatively new TB vaccine.
And by new, I mean, it was designed 20 years ago. And, the hope is that it'll be 50 percent efficacious. And lots of people off the record have told me things like, oh, we don't think it's going to work, but at least it'll set up the trial infrastructure for the next shot. I'm like, what if there is no next shot?
I think in TB in particular is a wonderful illustration of this problem where I think there should be a portfolio of a bunch of different candidates that are trying to do different things, elicit different kinds of immune response, and we should do a whole bunch of phase 1s. Right? And see what seems to work well in phase one, and then make decisions about what to put 500 million into.
And by we, I mean the whole community, right? The scientific community, the public health community. In fact, what is happening is a bunch of candidates designed like 20 years ago, which happen to have phase one data are being rushed into these extraordinarily expensive phase two, phase threes, instead of testing a broad portfolio of strategies upfront.
[02:01:46] Abhi: Why do you think, I guess like we have like mildly touched on this in the past, but it is surprising that like over 30 years, no one like mentally updates, like maybe this doesn't work. Is there just like, you know, like what's the cultural problem that's causing this?
[02:01:58] Soham: I think the argument that they would make is like there's no good empirical evidence that antibodies would be sufficient or important.
And they're not wrong about that, but you know, like what you've been trying for a long time hasn't been working.
[02:02:09] Abhi: I'm curious, like why do you, why do you think antibodies are like the answer to this?
[02:02:13] Soham: Well, I, you know, I speak not primarily my own opinion here, but, you know, research that, I think two branches of research that have sort of convinced me that there's something to be investigated.
I'm not saying it's going to work, but we should try it, right, is my main point. One, we know for, we know that there are antibodies because people have done this really, like, Babak has done this work. We know there are antibodies, against TB, which you can get even from, from patients, right? That do something.
They're functional. They're able to like, you know, do something to, to the pathogen, right? Which is not something that people thought was possible for a long time because it's an intracellular pathogen, right? In many ways. We also know, that, all the, as I said, all vaccines basically that are currently approved that actually are efficacious seem to work primarily using an antibody based mechanism.
Why would TB be any different? Right? And again, intracellular pathogen, I get it. But maybe, if you have an antibody response, you can stop the pathogen before it's able to invade the cells and take up residence. Maybe, you know, there are some antigens which which end up on the cell surface, even off the, you know, even off the, the cell that's infected with the pathogen, right?
Maybe there, there's another mechanism, which is the TB could be latent, could be inside the cells, but it doesn't matter. Because if you have the antibodies, when it tries to come out and replicate, something kills it, right? Exactly. And that's good enough, right? So, I think it should be investigated, right?
But I think there's, there's a certain, and you know, I'm not an expert in this field. I haven't been in this field for 30 years, but it doesn't seem like anyone is working on this particular vaccine design approach. There may be a few people, right? And so it seems to me that that's under invested, whereas the approach that hasn't worked for a very long time is very over invested.
And you know, maybe, maybe a good analogy to this is like all this like Alzheimer's plaque stuff, right? Like, it seems like there are these, you know, these, these dogma type things that happen in scientific fields. And then people just don't update for a very long time, right? Even if there's no good evidence that your approach is working, they're deathly afraid of other people getting resources to try a different approach.
Maybe because they think it won't work, but perhaps more insidiously, maybe some people are afraid that it will.
[02:04:33] Abhi: It's, it's interesting that, because like my, my initial assumption with like why Alzheimer's research has been stagnant for so long is like, almost they had too much money to play around with.
And so like they like they had this hypothesis. This hypothesis had money already behind it. So they just kept pushing money toward, like they were happy to pursue the hypothesis because there was money there. And so I assumed like because there's not that much money in vaccinology, there'd be more emphasis on like creativity or like in just trying things.
It seems like that's still not the case.
[02:05:00] Soham: I think, like, kind of the way I model this, and I could be wrong, is, like, in fields where there's, like, a ton of money, you do end up with more creativity because there's some, like, marginal money that gets just thrown at random stuff. That's not the case in vaccinology.
There's less money, and so it just, it, it becomes really, like, focused on, I think the money also gets deployed in big chunks, rather than, like, okay, HIV vaccines, so crazy over invested in, okay? The drugs work really well, now there's this new thing that is like a long acting antiviral that works for like 365 days or something and like 99 percent efficacy.
HIV is the hardest vaccine design problem. But there's like a hundred times more investment, maybe more, maybe it's a thousand times in HIV vaccinology, there are whole international organizations, like IAVI, the International AIDS Vaccine Initiative, that exist only basically to make HIV vaccines, right, That are invested in hundreds of millions up to billions of dollars, whereas we're not investing that money in hep C and TB vaccines, in novel hep C and TB vaccines, even though, even hep C, forget TB, which kills a million plus people a year, right?
I love vaccinology, like I work on vaccinology, but it's just such a hard problem, and there's so many easier problems that we still haven't solved. Why don't we work our way up to it? Right? And I think at one point, there was a good argument. There were so many people dying of HIV. But people haven't updated to the basic reality that the pathogen just doesn't kill quite as many people anymore.
[02:06:21] Abhi: Yeah.
[02:06:22] Soham: Whereas like, these other pathogens, they really do.
[02:06:24] mRNA beyond infectious diseases
---
[02:06:24] Abhi: That makes sense. And kind of like, beyond infectious diseases as a whole, I remember like, during like, the heyday of Moderna, they had this whole pitch about like, we'll use this, like mRNA technology for a bunch of things beyond infectious diseases like
[02:06:37] Soham: And they're pivoting to be more of a cancer company now yeah .
[02:06:39] Abhi: And like given PopVax's platform capabilities, do you think about potentially expanding into these adjacent areas?
Partially because of the commercial viability or also partially because like the raw capability you think mRNA has.
[02:06:50] Soham: So, look, we're always going to be a vaccine company. We're not going to give up on vaccines because that's why I started the company. That's what we care about. And today we're a vaccine company. And I can say that with pride. We're a vaccine company, right? In five years, if things are going well, we should not just be a vaccine company.
We want to be a company that harnesses the full power of mRNA as a platform to test lots of different designs. Right, and protein design that is informed by in vivo immunology data or informed by at least, you know, extremely high quality in vitro immunology data to be able to design the best immunotherapies, whether that's vaccines for infectious diseases, vaccines for cancer, you know, mRNA encoded cancer protein therapeutics, et cetera. So I think cancer is the next obvious thing for us. I think autoimmune also is another space where autoimmune vaccines, you know, immunotherapies for autoimmune conditions, where we have a bunch of ideas, right?
[02:07:45] Abhi: Do you think Moderna's relative lack of success in those areas has more to do with Moderna rather than for there to be vaccines for cancer?
[02:07:57] Soham: I think Moderna is an exceptional company in many ways, and we stand on their shoulders. I think Moderna is not good at protein design.
[02:08:03] Abhi: Gotcha.
[02:08:03] Soham: And I think a lot of Moderna's problems come from Moderna being bad at protein design. So recently they had this RSVH MPV vaccine safety issue, if I'm, if I'm not mistaken.
And I think a lot of, you know, again, it's like antibody dependent enhancement of disease. I think a lot of these companies are not natively protein design companies. Moderna is a mRNA delivery company. Their job, in some sense, was to get mRNA as a delivery platform to work. And then they just put a bunch of, you know, antigens that are what you would expect into their vaccine programs, and a bunch of protein replacement therapies, and a bunch of, now, antibodies that they're trying.
And then, you know, T cell cancer vaccines, which had been a concept in peptides, but now encoded into mRNA, leveraging, like, the simplicity of manufacturing of that platform, right? But they're not really design companies.
[02:08:50] Abhi: So in a certain sense, they got kind of lucky that COVID 19 was relatively easy to design for?
[02:08:56] Soham: Well, yeah, and they didn't, it wasn't their design, right? It was Jason McClellan's design out of NIH, and then they got sued, and they had to pay him a, I mean, they'd pay NIH a bunch of money because they used that design. So yeah, they got, I think we all got lucky. We got lucky, but two things. One is that COVID is an easy pathogen to make vaccines for.
Lots of different types of vaccines work for COVID. Which is why it's kind of ironic that the big vaccine companies, your GSK, Sanofi, Merck, which are the biggest vaccine companies, all failed at making COVID vaccines, which maybe tells you a little bit about where those companies are and their ability to develop novel products, right?
But, we'd also, and by we, I really mean, again, Jason McClellan, and his group, had done structural biology work on SARS one and MERS, and they had invented this stabilization, in the pre fusion conformation for the spike protein. And so that was directly applied by Moderna, by BioNTech, Pfizer, and by Novavax to, to their vaccines.
[02:09:56] What would you do with $100 million dollars?
---
[02:09:56] Abhi: Gotcha.
And I think like this may be like the last question I have. If you were given $100 million for PopVax to spend on like vaccine development, maybe like arbitrary basic research you want done. What would you do with that money?
[02:10:12] Soham: Like a hundred million philanthropic dollars.
[02:10:14] Abhi: A hundred million philanthropic dollars and yeah, equity free.
You can do no strings attached. You can do whatever you want with that.
[02:10:18] Soham: That's great. I already have this plan. So it's, we call it the million lives mission where we want to save a million lives per year with vaccines, which we can do with, if we have successful vaccines against three new, three pathogens where there are no existing vaccines, HCV, TB, and strep A.
And philanthropic dollars are great for this because, especially TB is a pathogen where lots of people think you can't make long term profits. HCV, I think, will actually be profitable, but, you know, it's a non trivial development process, something we're already working on, but speeding that up would be great.
And strep A is another pathogen where we think there's a market, but there's also a lot of developing world impact, right? And these pathogens kill lots of people, right? Two million, something like that, two million plus people per year. If we make vaccines that are reasonably effective and that are reasonably well distributed across the world, we can save maybe a million lives per year with these vaccines.
So what I would do with that money is I would scale up this feedback loop approach that we have, this machine learning feedback loop, to design libraries of immunogens to elicit specific antibodies that we know to work against these pathogens. In the case of TB, we'd have to do a more basic science effort.
To find, you know, these antibodies in the first place. So we, there's some very interesting study designs where people have done, where you can basically go to places like Bombay, where I grew up or in South Africa, where there's lots of active TB and you go to people whose houses have somebody with active TB, but who don't themselves have active TB.
And then you sequence out antibodies from those people, for example. And you can, those people are often people have been exposed to TB, but somehow have protected themselves against it.
[02:11:47] Abhi: Are there currently no like international efforts to collect that sort of data?
[02:11:50] Soham: For antibodies, not so much. So for hep C, there are some people doing this, but again, I think not a sufficient scale, right?
And so I would scale up those efforts as well. Yeah, I think, you know, one very valuable public good would make like a public antibody database, like a PDB esque database, that is then classified by disease phenotype and exposure to disease. So like, this is the antibody of somebody who like, had very mild disease or like asymptomatic, but was surrounded by people who had this pathogen.
As an example.
[02:12:21] Abhi: Yeah, I mean like, this cleanly feels like a huge public good to have. Has anyone tried to push for it and it just doesn't like work out in practice or just no one has really tried to take it to the next level?
[02:12:31] Soham: I don't think at scale anybody has tried to do that. NIH had a grant, program, I think like a couple of years ago for something that resembled this, but at scale nobody's really done this, I'm quite sure.
[02:12:41] Abhi: Okay.
[02:12:42] Soham: Okay. Thank you. So it should be done. I proposed it as an FRO a while ago. This feels very FRO y. But like, yeah, yeah, so maybe, maybe the FRO people will listen to this and think it's a good idea now. But, but I, yeah, a hundred million dollars, you know, this is what I would do with that.
And so I'd basically do the effort to sequence out the antibodies for TB and Hep C, I would, you know, design for the antibodies I want to elicit. And, in the case of strep A, we want to, design for, the ability to basically prevent the pathogen from getting a grip, you know, in your, you know, in your nose or throat or, basically just, you know, prevent it from, from adhering to, to the cells in the first place.
So it can never really colonize, right? So, So, you know, we would basically design, to elicit antibodies that do those things. optimize in the feedback loop way that I told you and like really scale up the data points we could get using these, especially organoid models, which use human immune cells.
To be able to get maybe even tens of thousands of data points for each of these pathogens that go from immunogen design to elicited antibodies and, you know, both what is the sequence and what is the functionality, and use that to optimize to basically design vaccines to elicit the antibodies we want, which hopefully then we could take into humans, do phase one, phase two.
[02:14:05] Abhi: And the hope of this, like a data collection of people who have latent TB and have like potentially useful antibodies is that you have like a good, like you have a good idea of like what you want your elicited antibody to look like.
[02:14:19] Soham: Well, I mean, for TB, we would have to find what those would be, but for hep C, we have some idea of what we want them to look like.
[02:14:23] Abhi: Okay, but like the collection effort is so you can like for for TB, you can actually learn.
[02:14:27] Soham: Yes, we can learn that.
Yes, exactly right.
[02:14:28] Abhi: Yeah. All right.
[02:14:30] Soham: I think in 100 million, you could get those three vaccines through phase one.
[02:14:33] Abhi: Yeah, I mean, like, that sounds reasonable enough. especially in India.
[02:14:36] Soham: Yeah. So if you want to save 1 million lives per year and you have 100 million dollars
[02:14:43] Abhi: People will reach out to you. I think those are about all the questions I have.
Thank you so much for coming on today
[02:14:50] Soham: Thank you for doing this I really enjoyed it and I'm very excited to hear more podcasts that you do with much smarter people than me.




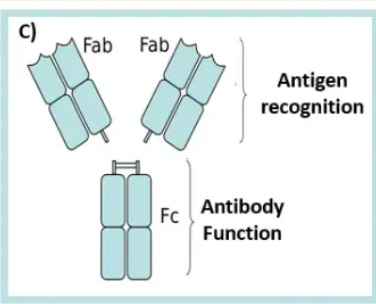


Share this post